The destruction of cells and their connections with complete dysfunction is called brain atrophy. Subatrophy of the brain is called partial local loss of brain function. With a disease such as cerebral atrophy of the brain, life expectancy does not change, since neurons die off gradually and, according to statistics, in most cases death occurs from other diseases. However, this disease is characterized by progression, leading the patient to dementia (acquired dementia).
Brain atrophy: causes, symptoms, diagnosis
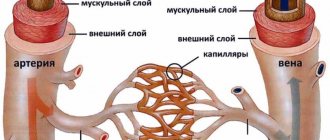
Atrophic changes in the cerebral cortex lead to the destruction of neural connections and a decrease in the activity of functional centers.
The condition leads to disruption of intracerebral metabolism, dementia, and the formation of a number of mental diseases (Alzheimer's, amyotrophic lateral sclerosis, dementia). Clinical symptoms depend on the type, stage, and degree of the disease. The multisystem form is accompanied by diffuse death of neurons and gradual loss of body functions.
Brain atrophy on MRI
Symptoms
Atrophic brain damage first manifests itself in barely noticeable changes: a person indulges in apathy and indifference, his aspirations disappear and lethargy appears, and his memory deteriorates. Previous skills are lost and new ones are difficult to acquire. Often there is a strong deviation from moral norms, irritability and conflict levels increase, sudden mood swings and depression occur.
The following symptoms are also observed:
- impoverishment of the vocabulary - a protracted selection of the necessary words to express ordinary realities;
- decreased brain activity;
- the disappearance of self-criticism and the ability to comprehend;
- sensitivity disorders, erectile dysfunction;
- deterioration of motor skills;
- parkinsonism.
Mental functions continue to deteriorate along with well-being. The ability to distinguish objects and use them decreases. The “mirror” syndrome is detected, in which the patient involuntarily repeats other people’s behavioral habits. Gradually, mental activity almost stops and complete incapacity sets in (stage of insanity), the personality disintegrates.
Specific symptoms of cerebral atrophy of the head depend on the involvement of different areas. For example, dysfunction of the frontal lobes negatively affects behavior and intelligence, and damage to the cerebellum affects motor skills, gait, speech and handwriting. If the nerve pathways are damaged, autonomic disorders may occur.
Causes of brain atrophy
After age 50, the risk of neurodegenerative conditions increases. Provoking factors increase the likelihood of the occurrence of a nosological form:
- Decreased kidney function (failure);
- Long-term increase in intracranial pressure (hydrocephalus);
- Frequent use of alcohol, drugs;
- Infectious damage to the cerebral cortex (retroviruses, poliomyelitis, encephalitis);
- Traumatic brain injury;
- Vascular diseases (thrombosis, atherosclerosis, aneurysm);
- Metabolic conditions;
- Mental illnesses - Alzheimer's, Itsenko-Cushing's syndrome, Parkinson's, Whipple, Gellervorden-Spatz.
Increases the likelihood of nosology - metabolic disorders, birth injuries, sexually transmitted infections, lack of B vitamins, folic acid.
The main causes of atrophy of the cerebral cortex
Scientific studies show a high probability of damage to cortical and subcortical structures in people 50-55 years old due to genetic predisposition. Cortical atrophy develops in patients suffering from hereditary Huntington's chorea.
Other reasons:
- Traumatic brain injuries accompanied by hematoma, neuronal death, and cyst formation;
- Chronic alcoholism, drug addiction, and the use of certain medications reduce the thickness of the cerebral hemispheres and subcortical sphere. Long-term alcohol intoxication disrupts intracellular metabolism and ensures the gradual death of neurons;
- Chronic cerebral (brain) ischemia is caused by vascular diseases (atherosclerosis, hypertension). Lack of oxygen contributes to irreversible tissue death;
- Congenital hydrocephalus in newborns leads to increased intracranial pressure and atrophy of the brain matter;
- More than seventy percent of cases of the disease in people over 55 years of age are due to neurodegenerative diseases - Pick, Lewy, Alzheimer's, Parkinson's. Nosologies form senile dementia.
Less common etiological factors of nosology are hypoxia of newborns, hydrocephalus, multiple congenital cysts in a child.
Causes of cerebral atrophy in newborns
The main etiological factor in reducing the thickness of the hemispheres of newborns is intrauterine hypoxia and problems during childbirth. Damage to the baby's head when passing through the birth canal provokes a traumatic brain injury and contributes to the appearance of hydrocephalus (dropsy).
Causes of atrophic cerebral changes in newborns:
- Damage to the bones of the skull;
- Increased amount of cerebrospinal fluid (hydrocephalus);
- Intrauterine infections (cytomegaly, herpes, meningitis).
There are no effective treatments for neonatal atrophy. Timely detection using MRI allows you to prescribe maintenance therapy and reduce the progression of the disease. Moderate changes are correlated with drug therapy. The child will be able to attend kindergarten and study in a special school.
Autoimmune encephalitis
After talking about the special immune status of the brain, we will move on to the topic of autoimmune encephalitis - a group of diseases that are associated with damage to the membrane and intracellular structures of neurons by the body’s own immunity. In modern practice, autoimmune encephalitis is rarely diagnosed. This is explained by the fact that the first cases were described in detail only in 2005 [10]. It can be assumed that there are actually more cases of this disease than are recorded by specialists. Some patients with autoimmune encephalitis may be diagnosed with other disorders, such as schizophrenia or infectious encephalitis. This is due to the fact that doctors have little knowledge about this disease. Doctors diagnose only those diseases that they know. The more arsenal of diagnoses a doctor has in stock, the more accurate the diagnosis and the more correct the treatment.
Autoimmune encephalitis can be divided into two groups:
- diseases caused by activated T cells (something similar occurs in multiple sclerosis [11]);
- diseases that occur when antibodies act on intra- and extracellular components of a neuron, such as ion channels.
The first group of autoimmune encephalitis is characterized by cell damage by antibodies and activated T lymphocytes [12]. These encephalitis are more severe and require intensive therapeutic interventions, unlike representatives of the second group.
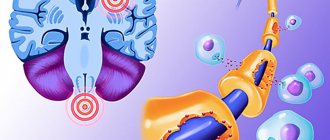
In the second group of encephalitis, neuronal damage is only “superficial” in nature. The effect of specific antibodies on surface-located structures leads to the “contraction” of receptors (in English this is called capping) and their subsequent internalization (capture) into the cytoplasm (Fig. 3) [13]. In addition, autoantibodies themselves can bind to receptors, blocking their work [14].
Figure 3. The changes that occur in receptors in autoimmune encephalitis are similar to the changes in synapses in myasthenia gravis. Normally (left side of the figure), the receptors are free and easily interact with the neurotransmitter acetylcholine. In case of illness, antibodies begin to affect the receptors, and their ability to bind to the neurotransmitter is impaired. The “clumped” acetylcholine receptors are gradually drawn into the cytoplasm, where they are destroyed.
www.memorangapp.com
Antibodies penetrate from the outside, from outside the BBB, or are produced by brain-infiltrated and activated B-lymphocytes [15]. In cases where antibodies are directed against intracellular structures, the attack on neurons is led by cytotoxic T lymphocytes. With the help of perforin and granzyme B, they damage the membrane of neurons, which leads to their death [16], [17]. The GEB, which we talked about above, in this light appears to be a reliable fortress wall protecting a quiet city that is no longer used to fighting. If a gap appears in the wall, the city will quickly fall: the nerve cells will be too sensitive to the effects of immune factors.
Looking for flags
Signs of autoimmune encephalitis are very varied, but three types of symptoms can be distinguished.
- Psychiatric symptoms: psychosis, aggressive actions, sexual disinhibition, panic attacks, obsessive actions, feelings of euphoria or fear.
- Motor symptoms: increased muscle tone and unevenness, muscle twitching and repetitive movements of the limbs.
- Seizures: generalized epileptic seizures, status epilepticus.
The initial manifestations of autoimmune encephalitis may be symptoms of a mental disorder: memory impairment, hallucinations or the appearance of delusional ideas. For example, with autoimmune damage to glutamate NMDA receptors, 80% of patients showed symptoms of mental illness, and more than 60% were initially hospitalized in psychiatric departments [18], [19]. Psychiatrists must be able to identify patients with autoimmune encephalitis or at least suspect this disease in order to promptly send the patient for appropriate examinations and consultations with other doctors.
Among the variety of symptoms that can be caused by different reasons, it is easy to get lost. The doctor needs at least rough guidelines to suspect the diagnosis of autoimmune encephalitis. As a result of the analysis of many cases of the disease, signs were identified that most likely indicate this diagnosis - the so-called “red flags” of autoimmune encephalitis [20]. These include:
- Lymphocytic pleocytosis or the appearance of oligoclonal bands on CSF electrophoresis.
- Epileptic seizures.
- Faciobrachial dystonic seizures.
- Suspicion of neuroleptic malignant syndrome.
- Abnormalities on MRI.
- EEG abnormalities.
In addition, the researchers also identified “yellow flags” - signs that should alert the doctor and make him think about a possible diagnosis of autoimmune encephalitis [20]. Yellow flags for autoimmune encephalitis are:
- Reduced level of consciousness.
- Violation of posture and movements.
- Instability of the autonomic nervous system.
- Focal neurological symptoms.
- Speech disorders (aphasia and dysarthria).
- Rapid progression of psychosis, despite treatment.
- Hyponatremia.
- Catatonia.
- Headache.
- The presence of other autoimmune diseases, including thyroiditis.
If a doctor sees symptoms from the group of “red flags” in a patient, the study authors recommend testing for specific autoantibodies for the timely diagnosis of autoimmune encephalitis. If a patient has symptoms from the list of “yellow flags,” the doctor should suspect the possibility of such a diagnosis and examine the patient more carefully to look for more reliable signs of autoimmune encephalitis.
Additional Arguments
In addition to the combination of previously described symptoms, the diagnosis of autoimmune encephalitis must be confirmed by laboratory tests and other diagnostic procedures [21].
For example, you can test for antibodies to specific receptors. In autoimmune encephalitis that affects glutamate NMDA receptors, an increase in the titer of antibodies to them can be detected. Interestingly, in encephalitis the amount of immunoglobulins of class G increases, and in schizophrenia - classes A and M [22]. Currently, a correspondence has been established between antibodies that affect specific neuron structures and the symptoms of encephalitis (Table 1). Magnetic resonance imaging (MRI) may show changes in brain structure, and electroencephalography (EEG) may show abnormal brain function in the case of autoimmune encephalitis. Table 1. Correspondence of symptoms and autoantibodies in autoimmune encephalitis [20]
| Structure to which an antibody is produced | Psychiatric symptoms | Other symptoms | Typical patient |
| NMDA receptor | Psychosis, schizophrenia-like disorders, catatonia, aggression | Epileptic seizures, dyskinesia, autonomic instability, speech and consciousness disorders | Young women, common association with ovarian teratoma |
| Caspr2 | Insomnia, panic attacks, depression, schizophrenia-like disorders | Morvan's syndrome, neuromyotonia, muscle spasms, fasciculations | Middle-aged and older patients, possible connection with thymoma |
| LGI1 | Amnesia and other memory impairments, confusion, depression | Limbic encephalitis, faciobrachial dystonic seizures, hyponatremia | Middle-aged and older patients, male to female ratio 2:1, possible association with thymoma |
| Glycine receptor | Behavioral changes, schizophrenia-like syndrome | Tight muscle syndrome, progressive encephalomyelitis with rigidity, myoclonus, hyperekplexia | Middle-aged and older patients, possible association with thymoma and lymphoma |
| Synaptic antigens (GAD) | Schizophrenia-like syndrome, autism, attention deficit hyperactivity disorder | Limbic encephalitis, stiff muscle syndrome, seizures, brainstem dysfunction, ataxia | Middle-aged and older patients, possible association with small cell lung cancer |
| Onconeural antigens (Yo, Hu, CV2, Ri, Ma2) | Behavioral disorders | Limbic encephalitis, cerebellar degeneration, sensory neuropathy | Elderly patients, often with malignant tumors |
In some cases, autoimmune damage to the central nervous system is caused by the development of paraneoplastic syndrome, which may accompany the appearance of a malignant tumor in the body. In paraneoplastic syndrome, the tumor can begin to independently produce hormones and hormone-like substances, disrupting the regulation of various processes in the body. In addition, paraneoplastic syndrome can manifest itself in the form of an autoimmune disease, for example, autoimmune encephalitis.
The pathogenesis of autoimmune encephalitis within the paraneoplastic syndrome is as follows. In a malignant tumor of a certain tissue, genes that are typical for it are expressed, as well as genes that are usually “silent” in it. Among the “silent” genes there may be those that are normally expressed only in the brain, under the protection of the BBB. Therefore, the resulting proteins are called cancer-neuronal antigens. During the maturation of the immune system, it becomes familiar with almost all the proteins of the body, but these proteins are hidden from it and, accordingly, are perceived as foreign. From the point of view of our immunity, there is no difference between them and bacterial antigens. The tumor begins to produce cancer-neuronal antigens, and the immune system recognizes them and produces autoantibodies specific to them. Through minimal gaps in the BBB, autoantibodies penetrate the central nervous system and begin to attack neurons [14].
For this reason, when diagnosing autoimmune encephalitis, special attention is paid to searching for a tumor in the patient’s body. Removal of the tumor in cases of paraneoplastic origin of autoimmune encephalitis will lead to a significant improvement in the patient's condition. While the tumor is in the human body, antibodies continue to be produced, and the patient’s condition worsens.
A blow to the immune system
Steroids and immunoglobulins administered intravenously are used as first-line drugs for the treatment of autoimmune encephalitis. Steroids have a powerful anti-inflammatory effect. They are capable of suppressing immune responses at a variety of levels. The use of steroids leads to a decrease in the synthesis of inflammatory mediators and stabilization of lysosome membranes that secrete inflammatory factors. In addition, under the influence of steroid hormones, the migration of monocytes decreases. Plasmapheresis, which cleanses the blood of “extra” antibodies, can also help.
If first-line therapy does not work, then switch to the use of rituximab and cyclophosphamide. Rituximab is a monoclonal antibody against the CD20 receptor, which is found on the surface of B lymphocytes [23]. B lymphocytes produce a variety of antibodies, including autoantibodies against nerve cells, and their destruction can lead to improvement. The CD20 receptor appears on the surface of normal B-lymphocytes and B-lymphocytes that have undergone the process of malignancy (malignancy). Due to this property, rituximab is effective in autoimmune diseases and B-cell lymphomas. The drug binds to the CD20 receptor on the surface of the lymphocyte and enables other components of the immune system to destroy the cell.
Cyclophosphamide has an immunosuppressive effect and is able to suppress the body's excessive immune response. After passing through the liver, cyclophosphamide is converted into several active metabolites that penetrate the cell and tightly connect the two strands of DNA. This prevents tumor cells or other rapidly dividing cells (such as B and T lymphocytes) from dividing. For this reason, cyclophosphamide is included in the treatment regimen for some autoimmune diseases. The subtlety of using steroids, cyclophosphamide and rituximab for autoimmune encephalitis is that these drugs can complicate the diagnosis of those tumors that are associated with the appearance of autoantibodies. In case of lymphoma, the use of these drugs can temporarily improve the condition, but when discontinued, the tumor will again “take up the old ways” and produce autoantibodies. Although lymphoma is not so often complicated by autoimmune encephalitis, this feature must be taken into account during therapy.
Using autoimmune encephalitis as an example, we can consider two of the most important trends in modern medicine. The first trend is the study of the influence of immune processes on the functioning of the human nervous system and attempts to intervene in this process in the treatment of neurological and mental diseases. We are now close to understanding that the brain is not just an electrochemical laboratory under the skull, but also a special immune world with its own rules. It is in our interests to learn these rules and begin to play by them to our advantage. The second trend is the biologization of psychiatry, the search for specific biological changes in the body that lead to mental health problems. Psychiatry is a part of medicine, a large branch where theoretical knowledge and its practical application are combined. Without a biological basis, medicine will cease to be scientific. If you ignore biological laws and knowledge within one of the medical specialties, then the same thing will happen to it. Psychiatry must become biological in order to remain scientific and fulfill its medical goals.
New Horizons
Recently, researchers and doctors have been paying more and more attention to innate immune responses during the development of neurodegenerative diseases (Alzheimer’s disease [24], Parkinson’s disease [25], Huntington’s chorea [26]) and mental disorders [27]. The accumulation of beta-amyloid, which itself is most likely involved in the immune responses of the central nervous system [28], is associated with an imbalance between its accumulation and elimination (removal). The latter process depends in part on how toll-like receptor 2 (TLR2) is expressed [29]. Mouse models have shown that the lower the level of TLR2 expression, the worse the removal of beta-amyloid [30]. Decreased elimination of beta-amyloid leads to its accumulation in nerve cells and subsequent disruption of their function and death. Studying the internal immune processes of the brain can become the basis for the search for new drugs for neurodegenerative diseases. Pharmaceutical companies are now testing monoclonal antibodies to treat Alzheimer's disease, but success remains modest.
Subatrophy of the brain - the first stage of senile dementia
Before clinical symptoms appear, subatrophic changes develop. There are no external symptoms. The condition is accompanied by a partial decrease in the function of a segment of the hemispheres.
Morphological types of subatrophy:
- Frontal;
- Frontotemporal;
- Parieto-occipital.
The first type is characterized by a decrease in mental activity, loss of speech and motor functions.
Damage to the frontotemporal areas leads to a decrease in a person’s hearing ability, communication functions are lost (difficulty communicating with other people), and the functioning of the cardiovascular system is disrupted.
Subatrophy reduces the volume of gray and white matter. Disturbances in conduction and motor function and fine motor activity occur.
Literature
- Craig L Hyde, Michael W Nagle, Chao Tian, Xing Chen, Sara A Paciga, Jens R Wendland, et al.. (2016). Identification of 15 genetic loci associated with risk of major depression in individuals of European descent. Nat Genet
.
48 , 1031-1036; - Novel study method identifies 15 genomic regions associated with depression. (2016). Science Daily;
- Code of Life: Reading does not mean understanding;
- Genetics of psoriasis: immunity, skin barrier function and GWAS;
- GWAS and psychogenetics: consortia in search of associations;
- The mysterious genetics of the “mysterious skin disease” - vitiligo;
- Wei Cheng, Edmund T. Rolls, Jiang Qiu, Wei Liu, Yanqing Tang, Chu-Chung Huang, et al.. (2016). Medial reward and lateral non-reward orbitofrontal cortex circuits change in opposite directions in depression. Brain
.
139 , 3296-3309; - Depression's physical source discovered. (2016). Science Daily...
Features of cortical atrophy
The death of cortical cells begins in the frontal lobes, where the functional centers for controlling movement and speech are located. Gradually, atrophy spreads to surrounding structures. In older people, the pathology leads to senile dementia.
Diffuse cortical changes are accompanied by microcirculation disorders and progressive clinical symptoms. Fine motor skills of the upper limbs and coordination of movements are impaired. The pathological complex leads to Alzheimer's disease and senile dementia.
MRI of cortical atrophy shows a decrease in the size of the frontal lobes. If there are changes on both sides, the functioning of the internal organs controlled by the frontal lobes is disrupted.
Congenital cortical atrophy of newborns is localized on one side. Symptoms are mild. With the help of rehabilitation procedures, it is possible to socialize the child.
What to do to stop dying
If cell death has already begun, then it should be slowed down and stopped. This can be done with the help of drug therapy. Groups of drugs used in medicine:
- Antioxidants.
- Neuropeptides.
- Neurometaloliths.
- Vitamins and minerals.
There are radicals inside every brain cell. When there are too many of them, they simply kill this cell. Antidepressants cope well with this process. Unfortunately, people quickly get used to them and have significant side effects.
Neuropeptides, in turn, slow down the death process and manage healthy cells, as well as build new ones.
Many feel the effect after starting to take these groups of drugs, because this is a kind of prevention from unwanted complications when the disease has progressed far and it is unlikely that anything can be done to save the situation.
Clinical symptoms of multiple system atrophy
Diffuse neurodegeneration is accompanied by problems in the reproductive and urinary areas. Necrosis of many parts of the brain is simultaneously accompanied by a variety of clinical symptoms:
- Muscle tremors in Parkinsonism;
- Impaired gait and mobility coordination;
- Loss of erection;
- Vegetative-vascular disorders.
Before the advent of magnetic resonance imaging, early diagnosis of the disease was problematic. Only nuclear magnetic resonance verifies a decrease in the thickness of the brain parenchyma.
Clinical symptoms of brain atrophy
The manifestations of pathology are largely determined by the causes and provoking factors. Most older people have dementia, frontal lobe syndrome, and internal multiple organ pathology.
How does frontal lobe syndrome manifest?
- Lack of appetite;
- Loss of memory, intellectual activity;
- Frequent emotional breakdowns;
- Lack of communication with surrounding people;
- Irritability;
- Lack of self-criticism.
Psychoorganic syndrome is accompanied by cerebroasthenic disorders, affective disorders, and amnesia.
The patient lacks an adequate assessment of surrounding events and self-criticism. Primitive thinking appears, a one-sided representation of the essence of the detail. Speech reserve decreases, paramnesia appears.
Concomitant affective disorders lead to depressive syndrome and inadequate mental state. Tearfulness, touchiness, irritability, unreasonable aggression are typical manifestations of pathology.
Types and classification of brain atrophy
According to the degree of danger, there are two types of atrophic changes in the brain:
- Physiological;
- Pathological.
The first type is natural. Throughout human development, the death of the umbilical arteries and ductus arteriosus (in newborns) initially accompanies it. After puberty, the tissue of the thymus gland is lost.
In old age, degenerative changes in the genital area occur. In elderly people, cortical destruction and involution of the frontal part appear. The condition is physiological.
Types of pathological atrophy:
- Dysfunctional – develops with a decrease in the functional activity of the brain;
- Compression – provoked by increased pressure on brain tissue (hydrocephalus, hematoma, copious accumulation of blood);
- Ischemic (dyscirculatory) occurs due to narrowing of the lumen of the arteries due to atherosclerosis, blood clots, and increased neurogenic activity. Generalized cerebral hypoxia is accompanied not only by mental dementia and sclerotic intracerebral changes;
- Neurotic (neurogenic) is formed due to a decrease in the flow of nerve impulses to the internal organ. The condition is formed due to gradual hemorrhages, the presence of intracerebral tumors, atrophy of the optic or trigeminal nerve. Occurs during chronic intoxication, exposure to physical factors, radiation therapy, long-term treatment with non-steroidal anti-inflammatory drugs;
- Dishormonal - occurs against the background of endocrine imbalance in the ovaries, testes, thyroid gland, mammary glands.
Morphological types of brain atrophy:
- Smooth – the surface of the brain is smoothed;
- lumpy - uneven distribution of areas of necrosis forms a special structure;
- Mixed.
Classification according to the extent of damage:
- Focal - only isolated areas of atrophic damage to the cerebral cortex can be traced;
- Diffuse - spreads over the entire surface of the parenchyma;
- Partial – necrosis of a limited part of the brain;
- Complete – atrophic changes in white and gray matter, degeneration of the trigeminal and optic nerves.
The nature of morphological changes in the brain is revealed by magnetic resonance scanning. Scanning should be done after the first clinical symptoms appear.
How to save neurons
Nerve cells are restored - myth or reality? During the process of embryogenesis, all cells, tissues and organs are laid down and formed inside the body, in exactly this order. A huge number of brain cells are formed even before the birth of a child - he loses some at birth, another part during the first years of life, and so on throughout his entire life. Restoring nerve cells is a myth that can be turned into reality with little effort. These efforts consist not only in training memory and attention through various exercises, reading books, watching films, intellectual games, but also proper nutrition - it is the key to the health of the whole body.
The following factors can preserve, prolong life and restore neurons:
- Sufficient amount of oxygen . It's no secret that the brain feeds on oxygen, which means that for its proper and normal functioning there must be a lot of this element. Pure water, foods that stimulate the production of hemoglobin and the brain will be fed in the right amount.
- Vascular balance . Cholesterol plaques that attach to blood vessels disrupt the flow of blood, and therefore oxygen, and therefore the nutrition of the brain
- Vitamin and mineral complexes . In addition to the vitamins and minerals that enter the body with food, it is necessary to consume potassium, calcium, magnesium, phosphorus and iodine in courses.
- Emotional comfort . Of course, a favorable psycho-emotional background for a person is an important aspect in this process.
Forms of multiple system atrophy
The danger of multiple lesions of brain structures is determined by a complex of pathological damage from the hemispheres, subcortical formations, cerebellum, spinal trunk, and white matter. Concomitant changes in the optic nerve lead to blindness, and in the trigeminal nerve – disruption of the innervation of the face.
Forms of multiple system atrophy:
- Olivopontocerebellar – damage to the cerebellum with impaired mobility;
- Striatonigral degeneration – muscle tremors with manifestations of Parkinsonism;
- Shy-Drager syndrome – vegetative-vascular dystonia, decreased blood pressure;
- Kugelberg-Welander amyotrophy is brain atrophy with muscle wasting and hyperplasia of connective tissue fibers.
Symptoms are determined by the predominant form of the lesion.
The main stages of atrophic brain changes
The disease has five degrees of progression. Based on clinical symptoms, it is possible to verify nosologies starting from the second or third stage.
Degrees of cortical atrophy:
- There are no clinical symptoms, but the pathology progresses rapidly;
- 2nd degree – characterized by a decrease in communication skills, lack of an adequate response to critical remarks, and an increase in the number of conflicts with other people;
- Lack of behavior control, causeless anger;
- Loss of adequate perception of the situation;
- Elimination of the psycho-emotional component of behavioral reactions.
Identifying any symptom requires additional study of the structure of the brain.
Principles of diagnosing atrophy
The initial stage involves taking an anamnesis, examination, and physical examination. The second stage is clinical and instrumental methods (ultrasound, CT, MRI of the brain, scintigraphy, PET/CT). Damage to the optic nerve is confirmed by ophthalmoscopy, tonometry, contrast CT or MRI angiography.
The best way to detect pathology of the soft tissues of the brain is MRI. The procedure must be performed several times (a month apart) to identify atrophy of varying depth and extent.
Magnetic resonance examination reveals the smallest local lesions and helps to correctly determine the degree of disease progression.
Causes of neuron death
The death of brain cells is a process that occurs throughout a person’s life. Doctors say that at a young age it is not scary, since the body is young - the hereditary factor plays a role in this case. Elderly people, especially after 55 years, encounter this phenomenon much more often and are diagnosed several times more often. This may be due to the following reasons:
- A heart attack is a process of disruption of blood flow to the main heart muscle.
- Stroke is a process of disruption of blood flow to the brain (cerebrovascular accident).
- Industrial emissions, working conditions, ecology.
- Previous injuries.
- Heredity and genetic predisposition.
- Pregnancy period (mother's use of drugs, cigarettes, alcohol), lack of professionalism of doctors - gynecologists and obstetricians.
There are many reasons for the spread of this phenomenon. Currently, MRI and CT scans are increasingly detecting serious changes in the neurons of the brain.