Paxil®
Children and teenagers (under 18 years old)
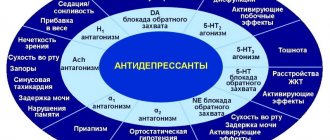
Treatment with antidepressants in children and adolescents with major depressive disorder and other mental illnesses is associated with an increased risk of suicidal ideation and behavior. In clinical studies, adverse events related to suicide attempts and suicidal ideation, hostility (mainly aggression, deviant behavior and anger) were observed more often in children and adolescents receiving paroxetine than in patients in this age group who received placebo. There are currently no data on the long-term safety of paroxetine in children and adolescents regarding the effects of this drug on growth, maturation, cognitive and behavioral development.
Clinical deterioration and suicide risk in adults
Young patients, especially those with major depressive disorder, may be at increased risk of suicidal behavior during paroxetine therapy. An analysis of placebo-controlled studies in adults with mental illness indicates an increase in the incidence of suicidal behavior in young patients (aged 18-24 years) while taking paroxetine compared with the placebo group (2.19% to 0.92%, respectively), although this the difference is not considered statistically significant. In patients of older age groups (from 25 to 64 years and over 65 years), an increase in the frequency of suicidal behavior was not observed. In adults of all age groups suffering from major depressive disorder, there was a statistically significant increase in the incidence of suicidal behavior during treatment with paroxetine compared with the placebo group (incidence of suicide attempts: 0.32% to 0.05%, respectively). However, the majority of these cases while taking paroxetine (8 out of 11) were reported in young patients aged 18-30 years. Data obtained from a study of patients with major depressive disorder may indicate an increase in the incidence of suicidal behavior in patients under 24 years of age suffering from various mental disorders. In patients with depression, worsening symptoms of the disorder and/or the emergence of suicidal thoughts and behavior (suicidality) may occur regardless of whether they are receiving antidepressants. This risk persists until significant remission is achieved. The patient's condition may not improve in the first weeks of treatment or more, and therefore the patient must be closely monitored for timely detection of clinical exacerbation and suicidality, especially at the beginning of the course of treatment, as well as during periods of dose changes, whether increasing or decreasing them. Clinical experience with all antidepressants suggests that the risk of suicide may increase in the early stages of recovery.
Other mental disorders for which paroxetine is used may also be associated with an increased risk of suicidal behavior. In addition, these disorders may represent comorbid conditions associated with major depressive disorder. Therefore, when treating patients suffering from other mental disorders, the same precautions should be taken as when treating major depressive disorder.
Those most at risk for suicidal ideation or suicide attempts are patients with a history of suicidal behavior or suicidal ideation, young patients, and patients with severe suicidal ideation before treatment and should therefore all receive special attention during treatment. Patients (and those caring for them) should be warned to monitor for worsening of their condition and/or the emergence of suicidal thoughts/suicidal behavior or thoughts of self-harm throughout the course of treatment, especially at the beginning of treatment, during changes in the dose of the drug (increase and decrease). If these symptoms occur, you should seek immediate medical attention.
It must be remembered that symptoms such as agitation, akathisia or mania may be associated with an underlying disease or be a consequence of the therapy used. If symptoms of clinical deterioration (including new symptoms) and/or suicidal ideation/behavior occur, especially if they occur suddenly, increase in severity, or if they were not part of the patient's previous symptom complex, it is necessary to reconsider the treatment regimen until drug withdrawal.
Akathisia
Occasionally, treatment with paroxetine or another SSRI drug is accompanied by the occurrence of akathisia, which is manifested by a feeling of internal restlessness and psychomotor agitation, when the patient cannot sit or stand quietly; with akathisia, the patient usually experiences subjective discomfort. The likelihood of akathisia occurring is highest in the first few weeks of treatment.
Serotonin syndrome/neuroleptic malignant syndrome
In rare cases, serotonin syndrome or symptoms similar to neuroleptic malignant syndrome may occur during treatment with paroxetine, especially if paroxetine is used in combination with other serotonergic drugs and/or antipsychotics. These syndromes are potentially life-threatening and therefore treatment with paroxetine should be discontinued if they occur (they are characterized by groups of symptoms such as hyperthermia, muscle rigidity, myoclonus, autonomic disturbances with possible rapid changes in vital signs, mental status changes including confusion, irritability, extremely severe agitation progressing to delirium and coma), and begin supportive symptomatic therapy. Paroxetine should not be prescribed in combination with serotonin precursors (such as L-tryptophan, oxytriptan) due to the risk of developing serotonin syndrome.
Mania and bipolar disorder
A major depressive episode may be the initial manifestation of bipolar disorder. It is generally accepted (although not proven in controlled clinical trials) that treating such an episode with an antidepressant alone may increase the likelihood of an accelerated development of a mixed/manic episode in patients at risk for bipolar disorder. Before initiating antidepressant treatment, careful screening should be performed to assess the patient's risk of bipolar disorder; Such screening should include a detailed psychiatric history, including a family history of suicide, bipolar disorder, and depression. Paroxetine is not registered for the treatment of depressive episodes in bipolar disorder. Paroxetine should be used with caution in patients with a history of mania.
MAO inhibitors
Treatment with paroxetine should be started cautiously no earlier than 2 weeks after stopping therapy with MAO inhibitors; The dose of paroxetine should be increased gradually until the optimal therapeutic effect is achieved.
Impaired kidney or liver function
Caution is advised when treating patients with severe renal impairment and patients with impaired liver function with paroxetine.
Epilepsy
As with other antidepressants, paroxetine should be used with caution in patients with epilepsy.
Seizures
The incidence of seizures in patients taking paroxetine is less than 0.1%. If a seizure occurs, treatment with paroxetine should be discontinued.
Electroconvulsive therapy
There is only limited experience with the concomitant use of paroxetine and electroconvulsive therapy.
Glaucoma
Like other SSRI drugs, paroxetine causes mydriasis and should be used with caution in patients with angle-closure glaucoma.
Hyponatremia
When treated with paroxetine, hyponatremia occurs rarely and mainly in elderly patients and is leveled off after discontinuation of paroxetine.
Bleeding
Bleeding into the skin and mucous membranes (including gastrointestinal bleeding) has been reported in patients taking paroxetine. Therefore, paroxetine should be used with caution in patients who are concomitantly receiving drugs that increase the risk of bleeding, in patients with a known bleeding tendency, and in patients with diseases predisposing to bleeding.
Heart diseases
Normal precautions should be taken when treating patients with heart disease.
Symptoms that may occur when treatment with paroxetine is stopped in adults
In clinical trials in adults, the incidence of adverse events with paroxetine discontinuation was 30%, while the incidence of adverse events in the placebo group was 20%.
Withdrawal symptoms reported include dizziness, sensory disturbances (including paresthesia, electric shock sensations, and tinnitus), sleep disturbances (including vivid dreams), agitation or anxiety, nausea, tremor, confusion, sweating, headaches, and diarrhea. These symptoms are usually mild or moderate, but in some patients they can be severe. They usually occur in the first few days after stopping the drug, but in rare cases they occur in patients who accidentally missed just one dose. Typically, these symptoms resolve spontaneously and disappear within 2 weeks, but in some patients they can last much longer (2-3 months or more). It is recommended that the dose of paroxetine be reduced gradually over several weeks or months before discontinuing it completely, depending on the needs of the individual patient.
The occurrence of withdrawal symptoms does not mean that the drug is abused or addictive, as is the case with narcotics and psychotropic substances.
Symptoms that may occur when treatment with paroxetine is stopped in children and adolescents
In clinical studies in children and adolescents, the incidence of adverse events with paroxetine discontinuation was 32%, while the incidence of adverse events in the placebo group was 24%.
Paroxetine withdrawal symptoms (emotional lability, including suicidal thoughts, suicide attempts, mood changes and tearfulness, as well as nervousness, dizziness, nausea and abdominal pain) were reported in 2% of patients during paroxetine dose reduction or after its complete discontinuation and occurred 2 times more often than in the placebo group.
Bone fractures
Based on the results of epidemiological studies of the risk of bone fractures, a connection between bone fractures and the use of antidepressants, including the SSRI group, was identified. The risk was observed during the course of treatment with antidepressants and was greatest at the beginning of the course of therapy. The possibility of bone fractures should be taken into account when prescribing paroxetine.
Tamoxifen
Some studies have shown that the effectiveness of tamoxifen, measured as breast cancer recurrence/mortality ratio, is reduced when coadministered with paroxetine, as a result of irreversible inhibition of CYP2D6. The risk may increase with coadministration over a long period of time. When treating or preventing breast cancer, the use of alternative antidepressants that do not affect CYP2D6 or have a lesser effect should be considered.
Impact on the ability to drive vehicles and operate machinery
Clinical experience with paroxetine suggests that it does not impair cognitive and psychomotor function. However, as with treatment with any other psychotropic drugs, patients should be especially careful when driving a car and operating machinery.
Although paroxetine does not increase the negative effects of alcohol on psychomotor functions, it is not recommended to use paroxetine and alcohol simultaneously.
Instructions for use of Paxil
The tablets are taken orally once a day during meals, in the morning. The dosage depends on the diagnosis, the severity of symptoms and the individual characteristics of the patient, determined by the attending physician. The following regimens for taking Paxil are possible:
- Depression: the daily dose is 20 mg per day. If indicated, it is possible to increase the dosage daily by 10 mg every day up to a maximum daily dose of 50 mg. After 15-25 days of use, the dosage is adjusted depending on the clinical picture.
- OCD, panic disorders: 40 mg per day; weekly the daily dose is increased by 10 mg (maximum dose is 60 mg/day). The duration of treatment is from 3 to 6 weeks.
- Generalized anxiety disorder, social phobias, post-traumatic stress disorder: 20 mg per day, if necessary, the dosage can be increased by 10 mg every 7 days to a maximum dose of 50 mg.
Paxil withdrawal syndrome
To avoid relapse of the disease, discontinuation of the drug Paxil is carried out gradually, reducing the daily dosage by 10 mg every seven days. If withdrawal syndrome occurs (sharp deterioration of the condition and return of the original symptoms), the drug is resumed with possible adjustment of the daily dosage. Therapy is continued for 5-21 days, then daily doses are reduced at a slower rate (the dose is reduced by 10 mg every 14-20 days). Withdrawal syndrome may be accompanied by the following symptoms:
- insomnia;
- anxiety;
- increased emotional arousal;
- dizziness;
- confusion.
Drug interactions
Paxil is not recommended for co-administration with monoamine oxidase inhibitors and for 2 weeks after the end of the course. Combined use with thioridazine increases the concentration of thioridazine in plasma (dosage adjustment is necessary). The drug enhances the effect of ethanol-containing drugs and drinks, reduces the effectiveness of Digoxin, Tamoxifen. Cimetidine increases the activity of paroxetine. Taking Paxil together with coagulants and antithrombic agents increases the intensity of bleeding.
Paxil price
Paxil can be purchased at a pharmacy with a doctor's prescription. Price range for all forms of release of the drug:
| Release form | Price range, in rubles |
| Film-coated tablets 20 mg No. 10 | 215-341 |
| Film-coated tablets 20 mg No. 30 | 625-755 |
| Film-coated tablets No. 100 | 1521-2246 |
Side effects
A course of treatment with the antidepressant Paxil can cause side effects and negative reactions from the endocrine, immune, reproductive, cardiovascular and central nervous systems, and digestive disorders. In these cases the following are observed:
- decreased appetite;
- metabolic disorders - diarrhea, constipation, nausea;
- from the respiratory system - yawning;
- increased levels of liver enzymes (in rare cases accompanied by the development of hepatitis or jaundice);
- drowsiness or insomnia;
- clouding of consciousness;
- sinus tachycardia;
- asthenia;
- visual impairment, development of glaucoma;
- decreased secretion of ADH (antidiuretic hormones);
- internal hemorrhages in the skin and mucous membranes;
- decrease or increase in blood pressure;
- increased sweating, skin rashes, urticaria, in rare cases - swollen lymph nodes, angioedema;
- sexual dysfunction;
- hyperprolactinemia;
- galactorrhea;
Overdose
Exceeding the dosage is accompanied by tachycardia, nausea and vomiting, agitation and increased excitability, changes in blood pressure, convulsions, dilated pupils, and fever. Cases of falling into a coma have been reported. When taken together with psychotropic drugs and alcohol, death may occur (very rarely). If an overdose is established, gastric lavage, activated charcoal, and maintenance therapy are prescribed. There is no specific antidote.