Treatment of post-traumatic sensory neuropathy
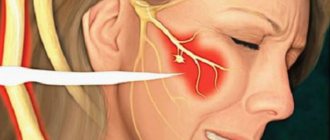
The most problematic result of dental surgery, local anesthesia or endodontic treatment is damage to the trigeminal nerve with subsequent dysfunction, most often of the inferior alveolar (IAN) and/or lingual nerve (LN). Altered sensations and pain in the orofacial area associated with damage to the branches of the trigeminal nerve can interfere with pronouncing sounds, eating and drinking, kissing, shaving, applying makeup, brushing teeth, and even working and sleeping.
Treatment of post-traumatic sensory neuropathy depends primarily on the causes and mechanisms of nerve damage and, most importantly, on the time after injury until treatment begins.
The trigeminal nerve is the largest peripheral sensory nerve in the human body, which is represented by more than 40% of the sensory cortex, so making the only correct decision about therapeutic tactics for a clinician after its injury is always a difficult task.
The most commonly iatrogenically injured branches of the trigeminal nerve are the inferior alveolar nerve (IAN) and the lingual nerve (LN).
The lingual nerve is located freely in the soft tissues, so the main causes of its damage are the removal of third molars and local anesthesia.
The inferior alveolar nerve is located in the bony canal of the mandible and the main causes of its damage include implantation, apical infection, endodontic treatment, and periapical surgery.
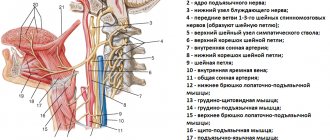
Rice. 1. Morphological preparation of the lower third of the face demonstrating the prepared lingual nerve, which runs deep in the soft tissues and is adjacent to the lingual surface of the mandible in the retromolar region.
Rice. 2. Schematic representation of the course of the lingual and inferior alveolar nerve.
After iatrogenic damage to the trigeminal nerve, the patient experiences not only impaired sensory function, but also a decrease in quality of life, psychological discomfort and social problems, the manifestation of which is more persistent in cases where the patient is older, and the time from the day of injury until the doctor recognizes the damage and makes therapeutic decisions more than 48 hours. It is also important to remember that post-traumatic consequences are more severe the more proximal the injury is to the cell body.
It is almost always difficult for patients to accept and cope with these negative consequences of oral and maxillofacial surgery, since third molar extraction or implantation is often an optional procedure, and the patient, choosing in favor of such surgical treatment, expects significant functional and/or aesthetic improvements.
There is still no consensus on treatment protocols for such injuries, but there is a clear understanding that the management and success of treatment of sensorineural deficits will be influenced by the mechanism and duration of nerve injury, injury-related clinical signs and symptoms, including psychological, functional or pain-related complaints of patients .
A recent Cochrane systematic review of treatment options for post-traumatic neuropathy resulting from dental procedures concluded that there is still a need for randomized controlled clinical trials to examine the effectiveness of surgical, medical and psychological treatments for iatrogenic injuries of the inferior alveolar and lingual nerves.
Coulthard P, Kushnerev E, Yates JM, Walsh T, Patel N, Bailey E, Renton TF. Interventions for iatrogenic inferior alveolar and lingual nerve injury. Cochrane Database Syst Rev 2014 Apr 16;(4):CD005293.
The priority when managing these patients is to have an honest conversation with the doctor about whether the nerve damage is permanent or temporary. This will help the patient make responsible decisions about surgical treatment, but pain control and rehabilitation should be started as early as possible, taking into account the psychological problems associated with iatrogenesis of the injury.
There is a false belief that most trigeminal nerve injuries recover, when in fact, for example, lingual nerve injuries associated with the creation of a lingual surgical approach are restored after 10 weeks and in only 88% of cases.
Factors influencing neurological response to nerve injury
— Preoperative screening for neuropathic pain is necessary. Preexisting neuropathic dental pain (PDAP type 1), which exists before surgery, can be caused by many different systemic conditions, medications, and other lesions. It is critical that surgeons recognize presurgical neuropathic conditions because neuropathic pain does not respond to surgery and can often lead to worsening pain. In addition, poorly controlled preoperative pain and nerve damage can cause chronic postoperative pain.
— The main indicators for predicting chronic post-surgical pain are psychological factors, including the level of anxiety, neuroticism (a fundamental personality trait in psychology, characterized by anxiety, fear, rapid mood swings, frustration and a feeling of loneliness. It is believed that neurotic people cope worse with stress and are prone to exaggerate the negative side of a particular situation.), catastrophization and introversion. Thus, the doctor has the opportunity not to perform the surgery of choice (implantation) in such patients, but to decide in favor of an alternative treatment plan.
- The concentration of the anesthetic used is up to 2% lidocaine - the accepted standard, because higher concentrations have a greater neurotoxic effect, which can cause permanent neuropathy. Avoid using multiple (repeated) anesthetic blocks in the same area for the same reason.
— Preoperative medical examination should exclude the following diseases: Raynaud's disease, Erythromelalgia (Mitchell's disease), Irritable Bowel Syndrome (IBS), Migraines, Fibromyalgia.
—Location of surgery is another factor associated with neurological response. Trauma in the distal part of the jaw is more significant (for example, the area of the angle and ramus of the jaw) than in the area of the mental foramen, because the closer the proximal site of nerve injury, the higher the risk of damaging trigeminal ganglion cells and initiating retrograde differentiation effects into the central nervous system.
Thus, a thorough interview and examination of the patient, detailed pre-implantation planning based on CBCT data, appropriate visualization of the implantation plan and the use of surgical guides, selection of optimal implant sizes with extended safety zones, use of drill limiters and, of course, an experienced team of doctors who will carry out the implantation followed by early postoperative care, all of which will contribute to safer practice and optimized patient outcomes.
Management of patients with post-traumatic sensory neuropathy
Consultation with a patient with post-traumatic sensory neuropathy usually requires a minimum of 30–40 minutes of time.
- It is necessary to carefully and in detail collect a medical history, including: - date and origin of nerve damage; — patient’s self-assessment of neurosensory function in terms of changes in sensitivity: hyperesthesia, hypoesthesia, anesthesia; — patient self-assessment of neurogenic discomfort and regular pain: paresthesia, dysesthesia, allodynia, dysgeusia, ageusia (loss of taste); — psychological screening; — functional screening (impact on daily life).
- It is necessary to conduct a series of standardized tests of neurosensory functions, using mapping to assess the size of the neuropathic area, determine the nature of functional problems and assess the level of pain. Pain is assessed using a visual analogue scale, where 0 is no pain and 10 is the worst possible pain.
- Assess the percentage of the neuropathic area of the extraoral and intraoral dermatomes, where 100% of the extraoral dermatome - the entire extraoral area of the skin innervated by the inferior alveolar nerve on the damaged side is involved in changes, 100% of the intraoral dermotome - the entire mucosa in the area of innervation of the lingual nerve on the damaged side is affected.
- Inform the patient about the diagnosis, extent of injury, probable cause, and persistence of injury.
- Discuss possible symptomatic and etiological treatment strategies and expectations regarding such treatment. Inform the patient how his symptoms are explained by current understanding of neurobiology (eg, cold allodynia).
- The patient must be given the opportunity to communicate by telephone with the doctor.
It is important to differentiate post-traumatic neuropathy from trigeminal neuropathy associated with malignancy, multiple sclerosis, sickle cell disease, neurological disease, alcoholism, trauma, diabetes, HIV, postherpetic neuralgia, stroke, or chemotherapy.
Management options for patients with post-traumatic neuropathy will depend on understanding of the mechanisms and duration of injury, the symptoms identified, and the wishes of the patients.
Patient management strategies include:
- Comfort and observation.
- Early medical treatment (steroid hormones, vitamin B complex, and NSAIDs-nonsteroidal anti-inflammatory drugs) is aimed at minimizing the development of the inflammatory response to nerve damage and promoting its recovery. When local pain is present, there is evidence for the use of topical lidocaine 5% patch and local clonazepam injections. Botulinum toxin type A (Botox) injections for focal neuropathic pain have limited success.
- Psychiatric and psychological support in the form of cognitive behavioral therapy and based on the results of psychometric questionnaires, including the PainDETECT . It is important to reassure patients with permanent nerve damage that their condition will neither worsen nor improve, and that the condition does not predispose them to the development of any other pathology in the area. It is also very important to explain to the patient the pathological physiology of nerve fiber damage and honestly tell how difficult this condition is to treat.
- In cases of chronic pain, systemic administration of pregabalin, oxcarbazepine, venlafaxine or nortriptyline.
- Early or delayed surgical treatment, decompression or direct anastomosis.
- In cases of trigeminal neuropathic pain, alternative pain treatment strategies such as transcutaneous electrical nerve stimulation (TENS), acupuncture, low-level laser therapy, and myogymnastics may be effective.
- Speech therapy assistance.
- Observation and assessment of the dynamics of the condition according to the following results: reduction of pain, improvement of functionality and the patient’s ability to cope with iatrogenic post-traumatic neuropathy.
Typically, patients need a comprehensive approach to treating neuropathy, based on the pain, functional and psychological problems the patient is experiencing. Juodzbalys G, Wang HL, Sabalys G. Injury of the Inferior Alveolar Nerve during Implant Placement: a Literature Review. J Oral Maxillofac Res 2011 Apr 1;2(1):e1.
Clinical algorithm for determining post-traumatic neuropathy
Trigeminal Neuropathy Assessment Questionnaire
Important! Severe pain experienced during treatment may indicate a possible nerve injury.
- History of the initial onset of pain.
- Development of pain.
- Duration of pain.
- History of regular pain SOCRATES (Site, Onset, Character, Radiation, Associated signs, Timing, Exacerbating and relieving factors, Severity) - Localization, Occurrence (frequency of attacks), Character, Irradiation, Associated symptoms, Duration, Exacerbation and relief factors (what intensifies and relieves pain), severity of pain.
- Psychological screening.
- Functional screening (impact on daily life).
Mechanosensory tests (mapping the affected area)
A protocol for examining the dermatome to assess the extra-oral mechanosensory function of the alveolar branch of the trigeminal nerve.
Dermatomes are segments of skin into which the entire surface of the human body is divided in connection with its innervation by various roots of the spinal cord, in this case the trigeminal nerve.
Figure 7. Dermatomes of the branches of the trigeminal nerve
1. Logging the affected area.
Using surgical forceps, move from the normal to the neuropathic (altered) area, warning the patient that there may be increased sensitivity and/or decreased sensitivity. Mark an area on the patient's face with marker marks and take a photo. Assess the % or area of the extra-oral dermatome that is affected by neuropathy.
2. Recording the assessment of subjective sensations.
Press the surgical forceps or probe firmly (but not painfully) against the patient's arm several times at intervals (5 times per minute) while explaining that this is a "normal" subjective rating of function on a scale of 10 out of 10. Press with the same pressure on the unaffected side face or tongue and repeat the stimulation, explaining that it should be 10 out of 10. Then remove the forceps or probe and explain that the missing stimulation is 0 out of 10. Only then repeat the same actions in the area of neuropathy that you have already confirmed and noted markers, and ask the patient to report the stimulus level out of a possible 10 (if > 10 = hyperesthesia and
3. Recording the light touch assessment
To assess light touch thresholds, it is recommended to use a frayed cotton swab, repeating touches at intervals of 5 times per minute. First on the unaffected side and then repeating on the affected side, ask the patient to report the differences. If the patient is experiencing numbness, then the stimulation will have a reduced threshold for detecting light touch, however, if the patient suffers from hyperesthesia and possible allodynia (pain to touch), then this test can be very uncomfortable and irritating.
Dermatomal Involvement Map Interpretation and Neuropathy Assessment—Does the area of neuropathy correspond to the dermatomal area in which surgery was performed? — Dynamics of the area of the involved extraoral and intraoral area when mapping neuropathic areas (criterion reliability is low)
Important! Localized sensory neuropathy is not always present in patients, but there is almost always an area of abnormal sensation, and the patient's maximum pain is associated with the area of sensory deficit, that is, suffering from a combination of pain, numbness and altered sensation. This is an important diagnostic distinction for sensory neuropathy.
Subjective function
- Is neuropathy hyperesthesia or hypoesthesia?
- Thermal allodynia test is the occurrence of pain when exposed to heat or cold. Thermal allodynia, especially cold allodynia, is a feature in patients with trigeminal nerve damage.
- Thermal hyperalgesia test. Increased pain that occurs after a weak stimulus.
- Test "Direction of movement". The patient closes his eyes, the doctor uses a soft brush to determine the patient's ability to detect both the sensation and the direction of movement of the brush.
- Test for sensitivity to temperature stimuli. A cotton swab with cold test spray and a dental mirror handle heated to 43 - 45 ° C are used to determine the patient's ability to feel cold and heat. Alternatively, test tubes can be filled with hot (43 -45 °C) water and cold water.
There are WHO recommendations regarding which parameters of peripheral sensory consequences should be taken into account to predict the results of microsurgical restoration of a damaged sensory nerve. Zuniga JR and Yates DM adapted the recommendations to trigeminal nerve lesions. More details here:
Today, a guideline for mandatory X-ray monitoring after endodontic treatment and dental implantation surgery has already been adopted in order to indicate the relationship between the root and the root filling, as well as the proximity of the implant bed and/or the implant itself to the canal of the inferior alveolar nerve. Intraoral dental radiography is considered to be sufficient to detect iatrogenicity, although post-traumatic neuropathy is primarily a clinical diagnosis.
Therapeutic measures in case of damage to the trigeminal nerve branch
| Event | Doctor's actions |
| Any nerve injury event | Suspected or known damage to a nerve and/or branch of a nerve requires IMMEDIATE treatment for repair and recovery. |
| Implantation | If <24-48 hours after surgery and numbness or neuropathy persists after local anesthesia has worn off, consider implant removal. N. _ B. _ On the same day, DO NOT reimplant, move the implant coronally, or replace the implant with a shorter one. |
| Endodontic treatment | If <24-48 after the onset of neuropathy, consider: - or surgical extraction of the tooth and removal of the exposed endodontic material or instrument; - or save the tooth, perform root canal filling (IDC) and long-term irrigation with saline solution. |
| Wisdom teeth surgery – injury to the inferior alveolar nerve | If <2 weeks, surgery may be indicated if radiographic evidence of tooth root fragments (incomplete extraction) or IDC damage is indicated. |
| Wisdom teeth surgery - lingual nerve injury | If >3-6 months consider cognitive behavioral therapy or surgery. |
| Anesthesia-related nerve damage (compression and ischemia of the LN or IAN) | Only therapeutic conservative treatment. |
| Nerve damage associated with orthognathic surgery | Only therapeutic conservative treatment. |
| Nerve damage associated with mandibular fracture | Only therapeutic conservative treatment. |
Many patients with sensory nerve injury present with a combination of symptoms of pain, anesthesia, hypoesthesia, hyperesthesia, and neuropathic symptoms such as pins and needles, and other paresthesias. Although these are different sensations, patients usually experience a mixture of symptoms in different parts of the neuropathic area. For example, a patient with inferior alveolar nerve injury (IANI) may experience burning pain in the chin along with lip numbness.
Rice. 3. The diagram shows the intersection of different manifestations of nerve damage. Although pain and anesthesia or hypoesthesia are diametrically different sensations, patients with nerve damage always experience combined symptoms in different parts of the neuropathic region. There are always patients who will experience a mixture of all symptoms and these are usually the hardest patients to treat.
Important! In patients with neuropathy, neurosensory testing should be performed every 2-3 weeks to assess nerve recovery, which will be reflected in a decrease in the intensity of symptoms and a decrease in the area of dermotome and mucosa involvement. Neurosensory improvement most often occurs after two to three months.
If sensitivity has not improved significantly three to four months after surgery, the prognosis is usually poor. Zuniga JR, Yates DM. Factors Determining Outcome After Trigeminal Nerve Surgery for Neuropathic Pain. J Oral Maxillofac Surg 2016 Jul;74(7):1323-9.
Nerve damage is defined as permanent if treatment is not initiated within 48 hours of injury and/or symptoms do not improve >1 month after implantation or endodontic treatment and >6 months after extraction of third molars or for other reasons.
If there is a persistent large neuropathic area (>40% of the dermatome), then severe nerve damage can be considered, and the development of pain and/or hypersensitivity are major factors that impair daily functions such as articulation, eating and drinking, shaving or applying makeup, and cleaning teeth, sleep, etc. and are often the main reasons for a patient to seek treatment.
It is the prevention of damage to the branches of the trigeminal nerve that is the main task of the clinician, since no treatment tactics guarantee complete restoration of the damaged nerve fiber and its functions.
The main recommendation for the doctor is early recognition of the injury to the sensory nerve branch, honestly informing the patient about realistic expectations for his recovery.
Text of the book “Complete reference book of a neurologist.”
Damage to the entire brachial plexus is characterized by the following clinical manifestations: paralysis of the muscles of the entire upper limb of a peripheral nature, periosteal and tendon reflexes decrease or disappear, persistent pain appears that spreads throughout the arm, all types of sensitivity are impaired in the area of innervation of the brachial plexus nerves.
Damage to the supraclavicular part of the brachial plexus (Duchenne-Erb palsy). With this pathology, the axillary nerve, musculocutaneous nerve, and radial nerve are affected. In this case, the function of all of the above nerves, as well as the muscles receiving innervation from them, occurs. These muscles are the deltoid, biceps, brachialis, brachioradialis, and supinator. In case of damage to the supraclavicular part of the brachial plexus, abduction of the affected arm, elevation to the horizontal line and adduction to the face are impaired. There is a loss of the flexion-ulnar tendon reflex from the biceps brachii muscle, upon palpation there is a sharp pain in the area of the supraclavicular fossa, all types of sensitivity on the skin of the shoulder girdle are impaired. The muscles of the forearm and hand retain their functional ability.
When the subclavian part of the cervical plexus is damaged, Dejerine-Klumpke palsy develops. The cause of paralysis is dysfunction of the ulnar, radial and median nerves. This leads to movement disorders in the forearm, hand and fingers. There is a disappearance of tendon and periosteal reflexes in the upper limb. On palpation, sharp pain is noted in the area of the subclavian fossa, radiating throughout the arm. In addition, there is a violation of all types of sensitivity on the inner surface of the shoulder, forearm and hand of the radicular type.
Lumbar plexus lesions
Lumbar plexus (plexus lumbalis)
formed by the anterior branches of the four lumbar spinal nerves. The lumbar plexus is located behind the psoas major muscle, in front of the transverse processes of the lumbar vertebrae. The lumbar plexus gives rise to the femoral nerve, the obturator nerve, and the external cutaneous nerve of the thigh. These nerves innervate the lower limb. Damage to the lumbar plexus leads to dysfunction of the above nerves and, as a result, paralysis of the muscles of the lower limb develops. Damage to the lumbar plexus can occur as a result of birth injuries, pathological processes in organs located in the pelvic cavity, intoxication, etc. The clinical picture of lesions of the lumbar plexus includes symptoms of damage to the femoral, obturator and external cutaneous nerve of the thigh (see mononeuropathies of the lower limbs).
Sacral plexus lesions
Sacral plexus (plexus sacralis)
formed by the anterior branches of the fifth lumbar and four sacral nerves of the spinal cord. This plexus is the largest. The fibers of the nerves that make up it mix with each other and merge, forming the trunk of the sciatic nerve. The sacral plexus itself is located behind the piriformis muscle, anterior to the sacrum. The plexus is very close to the sacroiliac joint. The sacroiliac joint is often affected by pathological processes that can spread to the sacral plexus. Clinically, damage to the plexus is manifested by dysfunction of the nerves originating from it. These nerves are: sciatic nerve, tibial nerve, common fibular nerve, superior and inferior gluteal nerves, posterior cutaneous nerve of the thigh.
Throat-sacral plexitis
occurs as a result of damage to the nerves that make up the lumbar and sacral plexuses. Clinically, lumbosacral plexitis is characterized by the development of flaccid paralysis of the muscles that flex and extend the foot, the shin flexor muscles, and the hip adductors. In areas of the lower limb that receive innervation from the nerve fibers of the lumbar and sacral plexuses, a violation of all types of sensitivity is determined. There is loss of the Achilles tendon reflex and pain radiating to the lower limb. In the area of the lower leg of the foot, vegetative and trophic disorders are noted. The etiology of lumbosacral plexitis is varied. It includes a variety of pathological processes, such as inflammation and tumor formations of the abdominal and pelvic cavity organs, various infections and intoxications, and injuries during childbirth to the fetal head.
Lesions of the coccygeal plexus
Coccygeal plexus (plexus coccygeus)
formed by the anterior branch of the fifth sacral root and the coccygeal nerve.
The anal-coccygeal nerves originate in the coccygeal plexus, which innervate the muscles and skin of the perineum.
Damage to the coccygeal plexus can be provoked by a number of etiological factors, such as inflammation, tumor formation, infections and intoxications. Damage to the plexus is clinically manifested by pain and disruption of all types of sensitivity in the area receiving innervation from its nerves. Mononeuropathies
Mononeuropathies are damage to one peripheral nerve. The clinical manifestations of mononeuropathy depend on the function of the affected nerve. In case of damage to the motor nerve, exclusively motor disorders occur; in case of damage to the cutaneous nerve, there is a violation of all types of sensitivity. Most of all peripheral nerves are mixed in function, so when they are damaged, motor, sensory, and autonomic disorders are observed. Damage to the peripheral nerve causes the development of paralysis of the muscles receiving innervation from its fibers. The paralysis is peripheral. During the examination, the development of atrophy of paralyzed muscles is noted, reactions of muscle fiber degeneration occur, and the disappearance of reflexes carried out with the help of the affected nerve is noted. In typical cases, patients complain of pain along the nerve trunk. On palpation, pain is detected along the affected nerve. Peripheral paralysis that occurs with mononeuropathies is characterized by a number of symptoms. These symptoms are: hypotonia or atony of muscles receiving innervation from fibers of damaged nerves; reflexes carried out using the affected nerve are reduced or completely lost; muscle wasting or atrophy is observed; examination reveals the presence of neurogenic degeneration of muscle fibers with degeneration reactions. Damage to the peripheral nerves of the extremities is manifested by motor, sensory, and autonomic disorders, localized in most cases in the distal parts. With this pathology, patients complain of pain and paresthesia. An objective examination reveals a violation of all types of sensitivity of the “socks” and (or) “gloves” type, the presence of trophic changes in the skin of the affected limbs, as well as peripheral muscle paralysis and atrophy.
Mononeuropathies in most cases occur with compression lesions. These are the so-called tunnel syndromes. The cause of the development of tunnel syndrome is usually compression of the nerve trunk passing in the connective tissue canal or opening as a result of a decrease in their diameter, which may be a consequence of edema or hypertrophy if the nerve trunk is thickened. With the development of tunnel syndrome, mechanical compression of the nerve trunks occurs, and blood circulation in them is impaired. This is due to adjacent compression of the blood vessels accompanying the course of the nerve trunks. The cause of nerve compression may be a local or general pathological process. Local factors contributing to the development of mononeuropathy usually include increased tension in the ligamentous apparatus, as well as in the muscle fibers surrounding the nerve. Under the influence of a local pathological process, compression or stretching of the nerve occurs. This leads to the development of aseptic inflammation, the connective tissue elements of the walls of the canal containing the nerve undergo proliferation, and the development of osteofibrosis is characteristic.
Mononeuropathies of the upper limb
Axillary nerve damage
The axillary nerve is mixed in function. The motor fibers of the nerve innervate the deltoid and teres minor muscles. The sensory fibers of the axillary nerve are part of the superior lateral cutaneous nerve of the shoulder and innervate the skin of the outer surface of the shoulder. Damage to the axillary nerve is possible due to a number of reasons. In most cases, neuropathy of the axillary nerve is caused by trauma, such as a fracture or dislocation of the shoulder, a gunshot wound, prolonged compression of the nerve fiber, for example, with a crutch, incorrect position of the shoulder during sleep or anesthesia, etc. Clinically, damage to this nerve is characterized by the fact that the patient cannot move the arm to a horizontal level, which is explained by the development of paralysis and atrophy of the deltoid muscle. Looseness appears in the shoulder joint. The sensitivity of the skin of the outer surface of the upper third of the shoulder is also impaired.
Musculocutaneous nerve damage
This nerve is mixed in function. The motor fibers that make up the musculocutaneous nerve innervate the biceps, brachialis and coracobrachialis muscles. Sensitive nerve fibers innervate the skin on the outer surface of the forearm. The musculocutaneous nerve includes branches of the lateral nerve of the forearm. When the musculocutaneous nerve is damaged, atrophy of the biceps brachii, brachialis and coracobrachialis muscles is noted. There is a loss of the flexion-elbow reflex, as well as a violation of all types of skin sensitivity on the radial surface of the forearm and tenor.
Radial nerve damage
This pathology occurs more often than other lesions of the nerves of the upper limb. Nerve damage is caused by a number of reasons. The nerve can be affected during sleep if the patient sleeps on a hard surface with his arm under his head or under his torso. Basically, such a lesion occurs during deep sleep, which may be associated with intoxication or fatigue. The so-called “sleeping” paralysis. Also, neuropathy of the radial nerve can occur under the influence of prolonged compression by a crutch or tourniquet, as a result of fractures of the humerus. In some cases, radial nerve neuropathy can occur due to improper injection technique into the outer shoulder, which can occur if the nerve is abnormally located. In fairly rare cases, the factor that provokes damage to the radial nerve may be diseases such as influenza, pneumonia, typhus, etc. or intoxication, for example, alcohol or lead poisoning. The radial nerve is mixed in its function. The motor fibers included in its composition innervate the forearm extensor muscles, which include the triceps, ulnaris, hand muscles: extensor carpi radialis (short and long), extensor digitorum, extensor of the little finger, abductor pollicis longus, supinator . Innervating the above muscles, the nerve carries out the following motor functions: extension at the elbow joint, at the wrist joint, extension of the main phalanges of the fingers, abduction of the thumb, supination of the hand. Sensory fibers of the radial nerve are part of the following cutaneous nerves: posterior cutaneous nerve of the shoulder, inferior lateral cutaneous nerve of the shoulder, posterior cutaneous nerve of the forearm. Sensitive fibers of the radial nerve are involved in the innervation of the skin on the back surface of the shoulder and forearm, the radial side of the hand, the dorsum of the first, second and half of the third finger.
Clinical picture
When the radial nerve is damaged at different levels, various clinical manifestations will be observed. The radial nerve can be affected in the axillary fossa, the upper third, the middle third and the lower third of the shoulder. Damage to the nerve in the axillary fossa and the upper third of the shoulder leads to the development of paralysis of the muscles receiving innervation from its fibers. The following clinical picture is characteristic: there is a drooping of the hand when raising the arm, the first finger of the hand is brought to the second. The patient cannot straighten the forearm and hand, as the function of the extensor muscles is impaired. There is an inability to abduct the first finger of the hand and supination of the forearm. During the examination, loss of the ulnar extensor reflex is noted, as well as a decrease in the carporadial reflex. All types of sensitivity on the skin of the first, second and half of the third finger of the hand are impaired. Sensitivity disorders are most often expressed in the form of paresthesia. Damage to the radial nerve in the middle third of the shoulder is clinically characterized by preservation of forearm extension, as well as the ulnar extensor reflex. Sensitivity on the skin of the shoulder is preserved. An objective examination reveals all other symptoms of damage to the ulnar nerve. Damage to the radial nerve in the region of the lower third of the shoulder and upper third of the forearm is clinically characterized by the preservation of all types of sensitivity on the skin of the posterior surface of the forearm. Sensitivity on the skin of the back of the hand, as well as the function of the extensor muscles of the hand and fingers, is impaired or completely lost. Several diagnostic tests are necessary to determine whether there is radial nerve damage. If the patient's arms are stretched or raised upward, there is a drooping of the hand on the affected side. With the arm lowered, the patient cannot abduct the first finger and cannot supinate the hand. If you ask the patient to press his palms together and try to spread his fingers, then on the affected side the fingers will bend and slide along the palm of the healthy hand. The patient cannot abduct the first finger of the hand and simultaneously touch any plane with the back of the hand.
Ulnar nerve damage
Damage to the ulnar nerve ranks second in frequency among lesions of all nerves that make up the brachial plexus.
Etiology
In most cases, the cause of ulnar nerve neuropathy is compression in the elbow joint. This pathology can be observed in people when working with their elbows resting on a machine, desk, etc. Most often this occurs in exhausted people. The nerve may be compressed at the wrist level. In addition to compression, ulnar nerve neuropathy can be caused by a fracture of the internal condyle of the humerus or supracondylar fractures. In more rare cases, ulnar nerve neuritis occurs with a variety of infections, for example, typhus, typhoid fever, etc. According to the function performed, the ulnar nerve is mixed. The motor fibers that make up the nerve innervate the flexor carpi ulnaris, the deep flexor of the finger, and the adductor pollicis muscle. When contracted, the flexor carpi ulnaris flexes the hand and also abducts it to the ulnar side. The flexor digitorum profundus flexes the fourth and fifth fingers of the hand. In addition, this nerve innervates the muscles that adduct and abduct the fingers. The ulnar nerve also innervates the lumbrical muscles that extend the middle and distal phalanges of the fingers. Thus, the ulnar nerve performs the following movements: flexion and extension of the middle and distal phalanges of the fourth and fifth fingers, abduction and adduction of all fingers except the first, adduction of the thumb. In addition to the above motor acts, the ulnar nerve, together with the median nerve, performs flexion of the hand at the wrist joint, as well as flexion of all fingers of the hand except the first in the main phalanges.
The ulnar nerve includes sensory fibers that innervate the skin on the ulnar surface of the hand, as well as the skin of the fifth and part of the fourth fingers of the hand.
Clinical picture
Damage to the ulnar nerve results in the impossibility of palmar flexion of the hand. The ability to bend the fourth and fifth fingers is lost, the patient cannot bring and spread the fingers of the hand, as well as adduct the first finger of the hand. An objective examination reveals atrophy of the small muscles of the hand, hyperextension of the fingers in the area of their main phalanges, due to the preservation of the function of the radial nerve. The middle and distal phalanges of the fingers are in a flexed position. Externally, the hand of the affected limb takes the form of a “clawed paw”. The patient cannot flex the fourth and fifth fingers of the hand when clenching it into a fist. There is an inability to flex the distal phalanx of the little finger, as well as adduction of the fingers. Sensitivity disorder manifests itself in the form of numbness or paresthesia. Hypoesthesia or anesthesia of the skin of the palmar surface of the fifth and ulnar half of the fourth finger of the hand, as well as on the skin of the dorsum of the hand in the area of the fifth, fourth and half of the third finger may be observed. In addition to sensory disturbances, autonomic disorders in the form of cyanosis, impaired sweating and a local increase in skin temperature are sometimes observed in these areas. To detect the presence of damage to the ulnar nerve, namely its motor function, it is necessary to use a number of tests. The patient is asked to clench his hand into a fist. At the same time, he cannot bend the fourth and fifth fingers of the hand in the area of their distal phalanges. When trying to spread and bring together the second to fifth fingers of the hand with the palm positioned on a horizontal surface, the impossibility of this movement is noted. In a similar position of the hand of the affected upper limb, the patient cannot move the distal phalanx of the little finger. When trying to stretch a strip of paper by holding it between the thumb and forefinger of the hand, it is noted that it is impossible to press the paper with the thumb. This is a consequence of dysfunction of the adductor pollicis muscle. In this case, the patient holds the strip of paper by bending the distal phalanx of the thumb. The patient can perform this function with the help of the flexor pollicis longus, which receives innervation from the intact median nerve.
Median nerve neuropathy
Occurs in rarer cases than ulnar nerve injury.
Etiology
The etiological factors causing median nerve neuropathy are very diverse. These include various injuries of the upper limb, nerve damage in case of violation of the technique of intravenous injection into the ulnar vein, incised wounds of the palmar surface of the forearm above the radiocarpal joint, as well as overexertion of the hand of a professional nature. The function of the median nerve is mixed. The motor fibers of the median nerve innervate the following muscles of the upper limb: flexor carpi radialis, palmaris longus, flexors of the fingers (superficial and deep), flexors of the first finger (long and short), pronator teres and quadratus, abductor pollicis, and also muscle that opposes the thumb to the hand. Due to the fact that the median nerve innervates the above muscles of the upper limb, when they contract, the following types of movement are carried out: flexion and extension of the second and third fingers of the hand. In the area of their middle and distal phalanges, flexion of the first finger of the hand in the area of its distal phalanx, opposition of the first finger of the hand to the rest of the fingers, pronation of the forearm. Some types of movements are carried out by innervation of some muscles by the median nerve together with the ulnar nerve. These types of motor acts include palmar flexion of the hand, flexion of the fingers in the area of their proximal and middle phalanges, with the exception of the thumb. The median nerve includes sensory fibers that innervate the skin on the radial surface of the hand, the palmar surface of the first to fourth fingers of the hand, and the dorsal surface of the distal phalanges of these fingers.
Clinical picture
Damage to the median nerve leads to impaired pronation, impaired palmar flexion of the hand, as well as the first, second and third fingers. There is a violation of the extension of the second and third fingers in the area of their distal phalanges. The patient loses the ability to bend the first, second and third fingers of the hand when trying to clench his hand into a fist. Characterized by the impossibility of opposing the thumb to the rest. Sensory impairment is usually localized on the palmar surface of the hand, the same surface of the first, second, third and part of the fourth fingers, as well as on the dorsal surface of the distal phalanges of the second, third and partially fourth fingers of the hand. In most cases, pain of a causal nature is characteristic. An objective examination reveals pain on the palmar surface of the forearm. Atrophy of the hand muscles is detected, especially pronounced in the tenor area. As a result of atrophy, the first finger of the hand is aligned with the second finger in the same plane. The so-called “monkey’s paw” develops. Also, due to atrophy, there is an inability of the thumb to bend when trying to clench the hand into a fist. Vegetative-vascular disorders are observed, manifested in the form of pallor and cyanosis of the skin, brittle nails, the appearance of erosion and ulcers, impaired sweating, etc. In order to determine the presence of damage to the median nerve, several diagnostic tests are performed. When trying to make scratching movements with the index finger, pressing the palm to a horizontal surface, the impossibility of performing this movement is noted. When trying to clench the hand into a fist, it is noted that it is impossible to bend the first, second and partially third fingers of the hand in the area of their distal and middle phalanges (the so-called “hand of the prophet”). It is noted that it is impossible to oppose the thumb to the other fingers of the hand.
Treatment
Initially, conservative treatment methods are used, such as B vitamins, anticholinesterase drugs, physiotherapeutic treatment (massage and exercise therapy). If there is no positive dynamics within 1–2 months, it is necessary to resort to surgical methods of treatment.
Carpal tunnel syndrome
This syndrome involves entrapment of the median nerve in the carpal tunnel under the influence of an edematous and hypertrophied transverse ligament. This ligament is located between the ulnar and radial eminence of the wrist. Carpal tunnel syndrome can develop in a variety of diseases, such as acromegaly, hypothyroidism, amyloidosis, mucopolysaccharides, rheumatoid arthritis, etc. In the absence of various pathological processes in the carpal tunnel, compression of the median nerve does not occur, and tendon movement in this canal does not result in to nerve dysfunction. The palmar cutaneous branch arises from the median nerve. This branch is separated before the nerve enters the carpal tunnel. In the distal part of the canal, the cutaneous palmar branch of the median nerve divides into its terminal branches. The terminal branches innervate the abductor pollicis muscle, the oppons pollicis muscle, and the lumbrical muscles of the second and third fingers. Sensitive terminal branches innervate the skin on the dorsal surface of the first, second and third fingers in the area of their terminal phalanges, as well as the skin on the palmar surface of the first, second, third and half of the fourth fingers. The ulnar nerve is fixed in the transverse carpal ligament by fibrous bundles. When compression occurs in the carpal tunnel, not only the branches of the median nerve, but also the ulnar nerve are often damaged.
Clinical picture
Patients complain of the appearance of paresthesia most often in the area of the first, second and third fingers. Paresthesia usually occurs at night. Sometimes it covers all the fingers of the affected upper limb. At the beginning of the development of the disease, paresthesias are transient. As the disease progresses, paresthesias become permanent. Patients wake up at night from a feeling of numbness and swelling in the fingers. Sometimes pain appears in the hand area, radiating to the forearm, in some cases up to the elbow joint. Lowering the hand, as well as shaking it, helps reduce paresthesia. Increased pain is noted when the affected arm takes a horizontal position or when it is raised up. Increased pain under the influence of these movements is explained by a decrease in blood supply to the capillaries supplying the median nerve. Increased pain or its occurrence in the hand is noted during percussion or palpation of the transverse carpal ligament (positive Tinel's sign). A sharp increase in symptoms characteristic of carpal tunnel syndrome is observed when the hand is flexed for 2 minutes (Phalen's sign). Sensory impairment in the first three fingers of the hand is determined. In this case, there is a decrease in pain and temperature sensitivity. Weakness or atrophy of the muscle opposing the thumb to the hand is usually observed. Additional examination methods reveal signs of denervation of muscles receiving innervation from the fibers of the median nerve. Denervation has varying degrees of severity. In addition, it is possible to reduce the speed of impulses along the branches of the median nerve to the muscles of the hand. Many patients with a long course of the disease experience the appearance of cyanosis on the affected limb. Carpal tunnel syndrome is more common in women who do heavy manual labor.
Treatment
To achieve a positive therapeutic effect, it is first necessary to treat the underlying disease that caused the development of carpal tunnel syndrome. If the initial disease is hypothyroidism, replacement therapy is necessary. In this case, there is a rapid restoration of the lost functions of the affected nerve. In other cases, a variety of anti-inflammatory drugs are used, such as indomethacin, butadione and acetylsalicylic acid. In addition, novocaine and hydrocortisone are injected into the carpal tunnel. In cases of severe carpal tunnel syndrome, it is necessary to use surgical methods to decompress the nerve.
Ulnar nerve compression syndrome
The cause of this syndrome is compression of the ulnar nerve by the collateral ligament located between the olecranon process of the humerus and its medial supracondyle.
Clinical picture
Manifestations of ulnar nerve compression syndrome are paresthesia, as well as pain localized in the ulnar half of the hand and in the area of the fourth and fifth fingers. Weakness and, in some cases, atrophy of the small muscles of the hand are also noted. The deep branch of the ulnar nerve can be pinched at the level of the hamate or pisiform bones of the hand. This pathology will be clinically manifested by weakness of the interosseous muscles of the hand, the lumbrical muscles of the third and fourth fingers, the adductor pollicis muscle, and in some cases there is weakness of the adductor muscles of the fifth finger. With a more severe course of the syndrome, hypotrophy or atrophy of the above muscles of the affected limb may develop. There may be no pain associated with ulnar nerve compression syndrome. But if it occurs, the pain spreads to the entire hand of the affected upper limb.
Treatment
Therapeutic tactics for ulnar nerve compression syndrome include all the same principles as for carpal tunnel syndrome.
Mononeuropathies of the lower limb
Femoral nerve damage
The function of the femoral nerve is mixed. It consists of motor and sensory fibers. The motor fibers of the femoral nerve innervate a number of muscles of the lower limb. These muscles include the iliopsoas, quadriceps femoris, and sartorius muscles. All these muscles, when contracted, perform certain functions that are disrupted when the femoral nerve is damaged. The iliopsoas muscle performs flexion of the thigh at the hip joint. The quadriceps femoris flexes the thigh and also extends the lower leg. Contraction of the sartorius muscle causes flexion of the lower limb at the knee and hip joints. Sensory fibers of the femoral nerve are part of the anterior cutaneous branches of the femoral nerve and the saphenous nerve. The anterior cutaneous branches innervate the skin on the anterior surface of the lower two thirds of the thigh. The saphenous nerve innervates the anterior inner surface of the leg. Damage to the femoral nerve can be localized above or below the inguinal ligament. When the femoral nerve is damaged below the inguinal ligament, loss of the knee reflex, atrophy of the quadriceps femoris muscle, impaired leg extension, and disorders of all types of sensitivity in the area of dermatomes receiving innervation from the saphenous nerve are observed. When the femoral nerve is damaged above the inguinal ligament, all of the above symptoms are observed, which are accompanied by manifestations of impaired function of the iliopsoas muscle. The patient complains of difficulty while walking and running, which is associated with the inability to bring the hip to the abdomen. In addition, there is a violation of all types of sensitivity on the skin of the anterior surface of the thigh. In addition to all these clinical manifestations, Matskevich's symptom and Wasserman's symptom are observed. Matskevich's symptom is that when the lower leg of the affected limb is flexed, the patient, who is lying on his stomach, experiences pain in the anterior thigh. Wasserman's symptom is manifested by the appearance of pain when an outstretched leg is raised upward in a patient lying on his stomach. In this case, the pain is localized along the front surface of the thigh.
Clinical picture
Paresthesia of the thigh (Roth's disease).
With neuralgia of the cutaneous nerve of the thigh, or with its neuritis, the appearance of paresthesia in the skin of the thigh is noted. In most cases, this pathology is unilateral. Manifestations of the disease are attacks of paresthesia, manifested by a feeling of burning, numbness, tingling localized in the skin of the outer surface of the thigh. With prolonged standing or walking, paresthesia intensifies. Intensification of these sensations requires immediate stop and rest of the affected limb. If you continue to walk, paresthesia can turn into burning pain. Paresthesia attacks occur as a result of compression of the cutaneous nerve of the thigh by a bandage or belt near the anterior superior iliac bone. Most often, paresthesia of the thigh develops in old age, which is explained by difficulty in venous outflow, inferior capillary network and metabolic disorders. Usually the disease lasts a long time over many years.
Algorithm for patient management in the acute phase (first 30 hours)
There is a limited time window of up to 30-48 hours to make the most correct decision - to recognize the fact of damage to the alveolar and/or lingual nerve after implantation, endodontic treatment or removal of mandibular third molars.
Report by reference Khawaja N, Renton T Case studies on implant removal influencing the resolution of inferior alveolar nerve injury. Br Dent J. 2009 Apr 11;206(7):365–370 suggests that early removal of the causative implant (within the first 30 hours) results in maximum recovery, but the evidence remains weak.
Suggested protocol based on available evidence:
- Homecheck - The attending physician should contact the patient between 6 and 24 hours after surgery to determine the presence/absence of persistent partial neuropathy after local anesthesia has worn off. This contact is based on the relationship between doctor and patient, which is achieved through signed informed consent.
- Confirm the presence of neuropathy . If the neuropathy affects +/- most of the dermatome, severe nerve injury should be considered, which will be accompanied by neuropathic severe pain if not treated immediately.
- Admit the damage and apologize. An apology is NOT an admission of guilt.
- Additional CT scans or radiography are not necessary or valuable for decision-making in cases of implantation, but are absolutely necessary in cases of third molar extraction.
- Initiate treatment for implant-related nerve damage: - Consider removal of the implant within 30 hours of implantation or sooner; - early therapeutic treatment.
- Acute phase - nerve damage during surgery - Implant removal, immediate nerve restoration +/-.
Pharmacological therapy in the acute phase (first 30 hours) and intermediate phase (up to 4-8 weeks)
Pharmacological treatment of acute nerve fiber injuries includes the use of corticosteroids and non-steroidal anti-inflammatory drugs.
- Glucocorticosteroids – adrenocorticotropic hormone has been shown to inhibit central axon sprouting, reduce ectopic discharges in damaged sensory axons, and prevent neuroma formation. Seo K. et al.: Efficacy of steroid treatment for sensory impairment after orthognathic surgery. J Oral Maxillofac Surg 2004;62:1193-1201. Drug of choice - Dexamethasone - 8-12 mg/day for one week - Dexamethasone not only minimizes neuropathy after nerve injury when administered in high doses for one week after injury, but is especially recommended due to its significant anti-inflammatory effect compared to other corticosteroids. The recommendation is to prescribe a decreasing dose of dexamethasone (from high to low) for 5-7 days after a trigeminal nerve injury. Galloway EB, Jensen RL, Dailey AT, Thompson BG, Shelton C. Role of topical steroids in reducing dysfunction after nerve injury. The Laryngoscope 2000;110(10):1907-10. Kraut RA, Chanal O. Management of patients with trigeminal nerve injuries after mandibular implant placement. J Am Dent Assoc 2002;133:1351-1354.
- Nonsteroidal anti-inflammatory drugs (NSAIDs) are the best inhibitors of prostaglandin synthesis from damaged peripheral nerve endings. Prostaglandins released as a result of peripheral nerve injury sensitize peripheral nociceptor fibers and central neurons. Muller HW, Stoll G. Nerve injury and regeneration: basic insights and therapeutic interventions. Cun Opin Neurol 1998;11:557-559. Thus, maintaining therapeutic levels of NSAIDs in the blood as an adjunct to corticosteroids for 1-3 weeks after injury is very useful during the acute and intermediate phase of trigeminal nerve repair. Because any change in sensation may be caused by an inflammatory response, postoperative treatment with steroids followed by high-dose nonsteroidal anti-inflammatory drugs is given as soon as possible after any nerve injury. The drug of choice is Ibuprofen - 600 - 800 mg three times a day for three weeks (after finishing dexamethasone!). If necessary, two to three weeks after the injury, based on a repeat neurosensory examination, the doctor may prescribe an additional three weeks of taking NSAIDs if there are no signs of gastrointestinal disorders.
- Additional pharmacological agents - antidepressants, anticonvulsants, antisympathomimetic drugs, etc. Caution should be exercised with these types of pharmacological treatments, as they must be prescribed and monitored by a doctor who is familiar with the side effects of these drugs and has experience in treating nerve damage.
- Supportive pharmacological agents: - Neurorubin-Forte Lactab - one tablet twice a day for 4 weeks - contains high doses of vitamins B1, B6, B12, which play an important role in ensuring optimal metabolism in nerve cells. In high doses - has a weak analgesic effect; - Nucleo CMF Forte - one tablet twice a day for 20 days - Cytidine 5-monophosphate takes part in the synthesis of a complex of lipids that form the sphingomyelin membrane as the main component of the myelin sheath; - myogymnastics.
Treatment of neuritis of the axillary nerve
The main goal of treatment for neuritis of this nerve is to eliminate the cause of its occurrence. Therefore, first of all, the doctor must determine what problem provoked the inflammation of the peripheral nerve:
- If the cause is an infectious disease, then the patient should be prescribed antibacterial or antiviral drugs - the doctor will make the exact prescription after receiving the results of the relevant tests.
- In situations where the development of neuritis is provoked by disturbances in the functioning of the cardiovascular system, vasodilator drugs are prescribed.
- To cure neuritis of a traumatic nature, it is necessary to ensure complete immobilization of the injured limb.
If inflammation of the axillary nerve is caused by compression by crutches, then the patient should replace them with other means of similar use during recovery. If it is not possible to find such a replacement, the use of crutches should be minimized. In a situation where the disease is caused by a dislocation of the humerus, it is necessary to completely immobilize the injured area until the inflammatory process is completely relieved.
Next, anti-inflammatory drugs, B vitamins, analgesics, and decongestants are used. After a 2-week course of treatment, anticholinesterase medications and stimulants of biogenic processes are additionally prescribed.
A properly selected course of physiotherapy, which is added to the treatment plan from the end of the first week, is also of great importance for the successful treatment of neuritis. Among the methods of physiotherapy that are most effective in the treatment of neuritis are:
- electrophoresis with various drugs;
- ultraphonophoresis;
- impulse currents;
- UHF.
In addition, medical massages and exercise therapy can effectively and quickly normalize the functions of the affected area. Electrical stimulation is used to restore sensitivity and tone of damaged muscles. If neuritis due to a narrow musculoskeletal canal has taken the form of tunnel syndrome, then methods of direct injection of medications are used.
Algorithm for patient management in the late phase (after 3-7 days)
Within three to seven days after nerve damage, neuropathy is likely to be permanent and comprehensive treatment is indicated. Coulthard P, Kushnerev E, Yates JM, Walsh T, Patel N, Bailey E, Renton TF. Interventions for iatrogenic inferior alveolar and lingual nerve injury. Cochrane Database Syst Rev 2014 Apr 16;(4):CD005293.
According to many clinicians, in patients with IAN neuropathy in the late postoperative period, there is no indication for implant removal, since the implant itself no longer has a major role in repairing nerve damage and does not affect the development of symptoms.
Surgical treatment of the late phase.
Surgery is not indicated for patients whose nerve damage has resulted in partial neuropathy without pain and with preserved function, as any subsequent surgery will only worsen their condition and symptoms.
The success of surgical intervention performed according to indications is most predictable if such intervention is performed before the onset of Waller's degeneration, i.e., within three months after injury. Such early aggressive treatment may prevent the development of chronic refractory neuropathy.
However, studies report that neuropathic pain is NOT treated or improved by surgery, so if pain is the primary reason a patient seeks help, surgery is not indicated. Zuniga JR, Yates D. Factors Determining Outcome After Trigeminal Nerve Surgery for Neuropathic Pain. J Oral Maxillofac Surg 74:1323–1329, 2021.
Surgical tactics for patients with inferior alveolar nerve injury IANI:
- Inspection and sanitation of the inferior alveolar nerve canal.
- Decompression and removal of root fragments.
- Creation of a direct anastomosis.
- Autogenous and alloplastic nerve transplantation - these operations have demonstrated different results.
Zuniga JR. Sensory outcomes after reconstruction of lingual and inferior alveolar nerve discontinuities using processed nerve allograft—a case series. J Oral Maxillofac Surg 2015 Apr;73(4):734-744.
Surgical strategies for patients with LNI lingual nerve injury:
- Nerve decompression.
- Excision of scar tissue.
- Removal of neuroma.
- Creation of a repeat nerve anastomosis.
Pharmacological therapy in the late phase
Local therapy
On the skin - 5% lidocaine patch Versatisna 12 hours every 12 hours for 2-3 weeks. N. _ B. _ in cases of rash while using the patch, a decision must be made to replace it, for example, oral oxcarbazepine as a long-term treatment: Khawaja N, Yilmaz Z, Renton T. Case studies illustrating the management of trigeminal neuropathic pain using topical 5% lidocaineplasters. Br J Pain 2013 May;7(2):107-113. — for the oral mucosa – local oral anesthetic gels; - a combination of drugs for topical use: lidocaine and Tricyclics with or without capsaicin.
Systemic therapy
Neuralgic pain (continuous or periodic severe attacks)
- Pregabalin (Lyrica)
- Oxcarbazepine (Trileptal)
- Gabapentin (Neurontin)
- Lamotrigine (Lamictal)
Burning pain (usually constant or dysesthesia-induced)
- Tricyclic antidepressants (TCA) - Nortriptyline (Pamelor), Amitriptyline (Elavil).
- Serotonin and norepinephrine reuptake inhibitors (SNRIs) - Duloxetine (Cymbalta), Venlafaxine (Effexor).
- Tramadol is a very weak μ-opioid receptor agonist, induces serotonin release and inhibits norepinephrine reuptake. May be a useful adjunct for pain control purposes.
Center for Clinical Practice at NICE (UK). Neuropathic Pain: The Pharmacological Management of Neuropathic Pain in Adults in Non-specialist Settings [Internet]. London: National Institute for Health and Care Excellence, (UK); 2013 Nov.
Typically, a third of patients completely stop taking narcotic drugs due to their side effects, and only 15% of patients can take drugs from these groups systemically. About 18% of patients with inferior alveolar nerve injury (IANI) continue to use topical medications.
There are reports of the use of botulinum toxin A injections, but to date evidence of success is limited and the risk of facial paralysis is high.
Alternative pain management strategies may also be effective for neuropathic pain associated with damage to the trigeminal nerve branches:
- Transdermal electrical nerve stimulation (TENS), Bates JA, Nathan PW. Transcutaneous electrical nerve stimulation for chronic pain. Anesthesia 1980;35-817-824.
- Acupuncture, Sung YF, Kutner MH, Cerine FC. Comparison of the effects of acupuncture and codeine on postoperative dental pain. Anesth Analg Curr Res 1977;56:473-481.
- Low level laser therapy. Poole TE, Holland I, Peterson LJ. Clinical efficacy of low level laser treatment of oro-facial neurosensory deficits. J Oral Maxillofac Surg 1993;51(suppl. 3):182-186.
Some patients find relief from eating chili peppers.
Improvement criteria
One of the key criteria for improvement is a significant reduction in the size of the neuropathic area of the sensory dermatome, detected during control neurosensory examinations, which indicates spontaneous recovery of the nerve, which in most cases is partial.
In cases of lingual nerve neuropathy, a common finding is a residual neuropathic area on the lateral surface of the tongue adjacent to the teeth.
After using the 5% lidocaine patch, patients with IANI should experience an average reduction in mean pain levels on the PainDetect by half.
A decrease in the level of paresthesia is usually accompanied by an improvement in mechanosensory function, which ultimately leads to an overall improvement in the quality of life of such patients, however, it is very important to inform patients that treatment may not fully restore mechanosensory functions such as eating, drinking, articulation, etc., or special sensory functions, i.e. taste.
It is very important for patients with speech problems to have access to speech therapy.
A clinical psychologist can help patients cope with pain and allow the patient to lead as normal a life as possible, but it must be understood that a clinical psychologist cannot reduce the actual level of perceived pain.
Surgery in the late phase (3–6 months after nerve injury) should be the last option and only in cases where there is minimal or no dynamics of the large neuropathic area, poor mechanosensory function persists, and moderate to high levels of daily pain. After reparative surgery, exacerbation may occur in the form of increased pain from intermittent to constant, about which patients should be warned in advance and have the opportunity to agree or refuse the operation.
Diagnostics
To make a diagnosis, examination using palpation and a series of tests is important.
- Discriminative two-point test - the sensitivity of the branches is checked in turn and the reaction is compared.
- The sensory function of the radial nerve is checked by a discrimination test at two points and pricking the folds of the thumb.
- Motor branches are tested by joint extension.
- The sensitivity of the ulnar nerve is determined on the little finger; to control motor capabilities, the patient spreads his fingers with force.
- Additional tests to analyze ulnar nerve function include ring finger flexion and thumb adduction.
- Motor function of the median nerve is tested by resisting flexion of the wrist and index finger.
- A visual test of the sensitivity of the median nerve is a discrimination test with an attachment in the palm.
Referring patients to a neurologist
In certain situations, patients should be referred to a practicing neurologist with experience in the evaluation and treatment of nerve injuries:
- if you suspect a nerve has been crossed during surgery, immediately refer to a microsurgery specialist;
- If a patient is found to have dysesthesia or complete anesthesia at the first examination after surgery, the patient should be referred to a neurologist/microsurgeon, as immediate surgery may provide the best chance of neurosensory recovery.
If the patient has a small neuropathic area, mild anesthesia, or paresthesia, and the patient reports minimal impact on quality of life, adequate time should be allowed for eventual neurosensory recovery. If paresthesia does not improve within two to three months, the patient should be referred to a nerve injury specialist.
1. Reasons for violation of the integrity or conductivity of peripheral nerves
Impairment of the integrity or conduction of peripheral nerves
occurs as a result of domestic or work injuries, wounds (stabbing, gunshot) or sports training (sprains, fractures, dislocations).
There are frequent cases of birth trauma in newborns, aggravated by damage to the nerves of the limbs. Also complicated by impaired nerve conduction can be tumor diseases, hematomas, aneurysms, calluses and scars after suppuration. The nerve can be completely dissected, torn, compressed, or thinned. In the absence of proper surgical treatment at the site of injury, nerve cells are replaced by connective tissue. These scars that form impede nerve conduction. The limb loses sensitivity, loses functionality, this may be accompanied by chronic pain
.
Surgical treatment “neurolysis”
is used precisely in such situations. It is aimed at releasing the nerve from compression and restoring its conductivity.
A must read! Help with treatment and hospitalization!
General conclusions regarding treatment algorithms
- The mechanism of nerve injury and the timing of physician response/intervention are of paramount importance in making treatment decisions for trigeminal nerve injuries. There is only 24 hours from the time the nerve injury occurs before the condition is identified to maximize the chances of recovery.
- Surgical treatment may be indicated immediately after surgery in specific cases.
- Persistent central and peripheral changes occur in the nervous system 3 months after injury that are unlikely to respond to surgical treatment.
- Based on knowledge of cellular and biochemical mechanisms, the ideal time for nerve repair is the first 2–3 weeks after injury. It is during this period that maximum restoration of sensory functions occurs.
- Long-term treatment of patients mainly includes drug and psychological algorithms.
- Counseling and support is the most useful tool for treating patients with persistent sensory problems related to nerve damage.
- Drug symptomatic therapy is indicated for patients with chronic pain or discomfort, as well as for patients with anxiety and/or depression due to chronic pain. However, due to severe side effects from groups of drugs that are aimed at treating chronic pain, less than 8% of patients remain on medications.
- Painkillers for topical use - Versatis Lidocaine patch 5% - 12 hours after 12 hours.
- Systemic painkillers - Tricyclic antidepressants (Amitriptyline and Nortriptyline), Antiepileptic drugs (Pregabalin-Lyrica or Gabapentin) - blockers of neuropathic pain, but sometimes the patient has to pay for such relief with depression and even obsessive suicidal thoughts.
- Surgical interventions: - if the intersection of the nerve is reliably known - immediate microsurgery of the nerve fiber (application of a microsurgical suture) with subsequent monitoring of early signs of regeneration of the peripheral branches of the nerves; — removal of the implant within the first 24 hours (ideally); - revision of the damaged lower alveolar nerve no later than 4 weeks after the damage, for example, with fragments of the root of the third molar or endodontic mass removed to the nerve canal; — revision surgery of the damaged lingual nerve during the first 3 months after the injury.
What happens after nerve damage?
The interaction between peripheral sensory nerves and the central nervous system is extremely complex. It has been proven that minor trauma such as compression, concussion, contusion (bruise) or pinching can only lead to numbness, however, more serious trauma (chemical burn, partial or complete anatomical break) can lead to dysesthesia and/or neuropathic pain, which causes constant discomfort in patients (especially at night) and affects their quality of life.
- After damage to the fibers of the inferior alveolar nerve, degeneration develops within just a few minutes from the entire site of damage: retrograde towards the central nervous system and Wallerian towards the periphery.
- With acute compression, the axoplasmic flow is immediately disrupted, which will lead to a decrease in membrane excitability. If the compression is not removed = chronic compression, the development of axolysis and Wallerian degeneration begins, which in turn will lead to the development of fibrosis, the formation of neuroma and the progression of neuropathy.
- Even if the nerve fibers are partially interrupted, the possibility of spontaneous regeneration of the nerve remains due to the ingrowth of the axons of the terminal sections of its central segment into the peripheral section, provided that the implant is immediately removed. Between the interrupted nerve fibers, a linear array of Schwann cells is preserved, which significantly increases the nerve growth factor. In addition, the work of the gene encoding receptor mRNA is significantly activated and nerve regeneration is activated. These defense mechanisms and repair reactions can be completed within 2 to 3 weeks. This way, the nerve can reconnect fairly quickly unless there is severe neuropathy or complete rupture.
Although regenerative repair and nerve reconnection will be observed histologically, function is not completely restored. Reconnecting (reconnecting) the nerve does not mean healing, and under certain conditions can cause pain in patients.
The inferior alveolar nerve is a mixed nerve and is responsible for pain, touch, heat, cold, and pressure, although the mechanism of each sensation is different. An analogy can be drawn with an underground network of pipes in a big city, where gas, electricity, telephone, water and sewage are transported, but each pipe is separate and along its own route. If the nerve networks are damaged, the insulation of the structure is compromised, and when regeneration occurs, adjacent nerve fibers may be accidentally connected to each other. This means that impulses from peripheral nerves can be transmitted to the wrong destination, and from there to the central nervous system. If we take the pipeline analogy, it is as if groundwater and electrical cables severed by an earthquake were cross-connected.
Rice. 8. Inferior alveolar nerve. Mixed sensitivity. Each fiber is responsible for its own type of sensitivity.
This inappropriate connection is called “ephapsia” and was proven in laboratory animals back in the 70s of the last century.
Thus, after injury, nerve function is never completely restored, even if nerve regeneration is histologically completed through active repair.
Consider a case where nerve fibers designed to convey the sensation of cold accidentally become associated with heat fibers, which can compromise the patient's ability to respond to changes in temperature. There are countless numbers of nerve fibers, so injury to any particular nerve can result in connections to any number of other nerves depending on the severity of the injury. A large number of incorrect connections of nerve fibers leads to the formation of neuroma.
Neuromas often generate spontaneous discharge. This electrical impulse causes dysesthesia, and such patients usually complain of numbness, tingling or goosebumps on the skin.
Thus, the healing of a damaged nerve is an incredibly complex process, which is not limited only to the site of injury, but involves the entire system from the periphery to the central nervous system, in contrast to the healing processes of the mucosa or bone. Regressive changes in axons and myelin sheath, as well as nerve regeneration, affect all neurons not only histologically, but also molecularly and electrophysiologically. The forming ephaps creates a site of contact in which excitation from one cell to another is transmitted through electric current without the participation of mediators.
Rice. 9. Cross-excitation of adjacent fibers of cold and hot sensitivity due to ephaptic transmission of electrical impulses. It is possible for fibers of different diameters to interact (eg, cold and pain sensory fibers), with the signal propagating in both directions, which underlies stimulus-dependent pain symptoms and explains the abnormal perception of non-noxious stimulation in allodynia and hyperpathia.