The problem of treating patients who have suffered a spinal cord injury is one of the most difficult in the neurorehabilitation system. Such patients are psychologically and/or economically dependent on their loved ones and on society, since their movements are often significantly limited and the natural functioning of vital body systems is disrupted [1, 2].
Numerous modern studies have demonstrated great promise for the use of electrical stimulation of the spinal cord in motor rehabilitation of patients with long-term consequences of spinal cord injury. It has been proven that several years after the injury, after complete paralysis, patients can stand and walk independently after a course of rehabilitation using epidural electrical stimulation of the spinal cord [3-5]. It has been proven that the mechanism of action of epidural and transcutaneous electrical stimulation of the spinal cord (TESCS) is the same [6]: TESC, as well as epidural electrical stimulation, can be successfully used to restore the motor functions of patients paralyzed after a spinal cord injury due to its complete motor damage [7 -9].
A feature of most previous works that showed the capabilities of electrical stimulation of the spinal cord in restoring voluntary movements and independent maintenance of a vertical posture is that regular, often daily, stimulation effects and accompanying motor training lasted 5-9 months and all motor training was carried out by the efforts of 2- 3 methodologists in the presence of a physiologist or doctor [4, 5, 10]. That is, impressive results were achieved over a long period of time with the involvement of a large number of specialists. In fact, the vast majority of work in this area are expensive scientific studies conducted under specific experimental conditions on patients selected in accordance with the requirements of a particular study. It remains unclear how effective a course of electrical stimulation of the spinal cord is in restoring the motor activity of patients who have suffered a spinal cord injury outside of the limitations and experimental conditions.
Spinal cord injury is accompanied not only by motor impairments. It is important to evaluate the effectiveness and safety of electrical spinal cord stimulation procedures primarily for restoring bladder, bowel and sexual function in patients with spinal cord injury, since the greatest suffering in spinal patients is caused by violations of these functions [11]. Spinal cord stimulation with parameters used to restore motor function has previously been shown to affect urinary function [12, 13]. Recently published results of a urodynamic study showed that in patients with spinal cord injury who do not control bladder function, a single tESCS at the level of the Th11-12 vertebrae reduces detrusor overactivity, regulates detrusor-sphincter mismatch, increases bladder filling and facilitates urination [14]. Thus, it can be expected that a short-term course of electrical stimulation of the spinal cord, aimed at restoring motor activity, can have a positive effect on the rehabilitation of excretory functions.
The purpose of the study is to determine the effectiveness of using tESCS in combination with standard rehabilitation of patients who have suffered a spinal cord injury. An additional goal is to evaluate the effect of tESCS on excretory functions in patients with spinal cord injury.
Material and methods
The studies were carried out on the basis of St. Petersburg State Budgetary Healthcare Institution “City Hospital No. 40”. The purpose, objectives and protocol of the study were approved by the scientific problem committee (protocol No. 142). Informed written consent to participate in the study was obtained from patients.
Inclusion criteria for the study: age over 18 years, mid- and lower-thoracic level of injury, duration of injury more than 9 months, severity of spinal cord injury according to the American Spinal Injury Association (ASIA) scale - B or C, muscle tone of the lower extremities on the modified Ashworth scale - 2 or 3 points.
Exclusion criteria: severe concomitant diseases in the stage of decompensation, damage to the skin in the area where the electrodes are located, the presence of instability in the elements of the musculoskeletal system, mental illness.
The study involved 15 patients with late-stage spinal cord injury (Table 1;
Table 1. Description of study participants. Changes in motor activity indicators before and after treatment Note. * — patients underwent rehabilitation on an outpatient basis. Female patients are in italics, differences in indicators before and after the course of treatment are in background, positive dynamics are in bold, and negative dynamics are in regular font. patients are ranked within each group according to the duration of the period after injury). All patients underwent a standard course of rehabilitation treatment. The rehabilitation course consisted of physical therapy classes, mechanotherapy, including robotic therapy (Lokomat, Hocoma, Sweden), massage, and physiotherapy, performed daily, 10 procedures per course. The duration of the study was ~2 weeks.
The main group (Nos. 1-7 in Table 1) consisted of patients who agreed to undergo the TESCM procedure. They received a standard course of therapy and TESCM procedures. Patients in the control group (nos. 8-15 in Table 1) received only standard treatment.
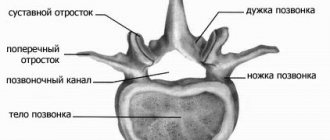
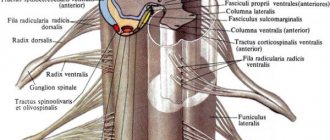
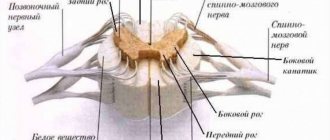
The BioStim-5 device (Kosima LLC) was used for ESSM. Electrodes (WFB02 QWER, China; BF4, LEAD-LOC, Inc., USA) with an adhesive conductive layer were fixed cutaneously. A stimulating electrode (cathode) in the form of a disk with a diameter of 2.5 cm was placed on the midline of the spine between the spinous processes at 3 levels: C5-6, Th11-12 and L1-2. Indifferent electrodes (anodes) were placed symmetrically over the iliac crests. Stimulation was carried out by rectangular pulses (monopolar and/or bipolar) with a duration of 1 ms, filled with a carrier frequency of 10 kHz. The magnitude of the current was selected individually so that it was not painful and at the same time caused contraction of the muscles of the lower extremities. The amplitude range of the currents used was 30–120 mA. During the procedure, the current value was gradually increased by 20-40 mA. The pulse frequency was 15–30 Hz. TESSM was carried out simultaneously with motor training on a simulator for active-passive rehabilitation of the upper and lower extremities (Thera vital, “Medica Medizintechnik”, Germany). The training regimen was selected individually depending on the functional abilities of the patients and the assigned rehabilitation tasks. Also, to improve statics, exercises were used while sitting on a chair, standing in a knee support with a gradual decrease in the area of support. When choosing the parameters of stimulating effects, we were guided by the results of using tESCS to regulate locomotor [8] and postural functions [10] in patients with vertebrospinal pathology.
The duration of the complex procedure was 30 minutes; there were 10 procedures per course, performed daily, 5 times a week.
Standard scales were used to assess the neurological status of patients before and after the course of treatment. To determine the severity of spinal injury in terms of sensitivity and muscle strength, the ASIA/ISNCSCI scale (International Standards for Neurological and Functional Classification of Spinal Cord Injury) was used. The Ashworth scale was used to determine muscle spasticity. The strength of the flexor and extensor muscles of the hips, legs and feet was assessed using the 6-point British Medical Research Council scale (Harrison scale) [15]. Also, the standard neurological examination included determining the level of hypo- and anesthesia, the presence of deep sensitivity and muscle-articular sensation. Patients filled out a urination diary, in which they noted the urge to urinate and its control. The amount of residual urine was monitored using bladder catheterization or ultrasound. All studies were carried out before the start of the rehabilitation course and immediately after its completion.
Statistical analysis of changes in muscle strength and sensitivity was performed using Student's t test and the nonparametric Wilcoxon test, comparing values for both legs of the patient without averaging them. Differences between indicators were considered statistically significant at p
<0.05 for each criterion. Other indicators were compared using the Mann-Whitney or Wilcoxon tests.
3.Preparation for surgery to implant electrodes
Since as a result of the operation a foreign body will enter the patient’s body and be placed for a long time, it is necessary to carry out preventive measures to eliminate the risk of infection and the development of other complications:
- examination of the patient’s general condition for tolerability of surgical treatment (cardiovascular and respiratory systems, immune status and underlying diseases);
- identification of possible latent infections by bacterial culture of scrapings from the nose, pharynx, perineum (Staphylococcus aureus is especially dangerous);
- antibiotic therapy (when identifying infections, the sensitivity of bacteria to different groups of antibiotics is simultaneously assessed, which is taken into account when choosing drugs);
- agreement with the patient on the possible localization of the subcutaneous generator.
About our clinic Chistye Prudy metro station Medintercom page!
results
The average age of patients in the control group was 27.9±3.80 years, in the main group - 29.9±8.07 years. The average period after injury was 2.8±2.21 and 1.9±1.06 years in the control and main groups, respectively. There are no statistically significant differences between the groups in these parameters. Spasticity scores in the control and main groups were 2.7±0.39 and 2.1±0.35 points on the Ashworth scale, respectively. Initially, according to the scores of muscle strength, sensitivity, and recorded indicators of the urinary system, the groups were homogeneous among themselves and did not have statistically significant differences (see Tables 1 and 2).
Table 2. Changes in sensitivity and excretory function before and after treatment Note. * — 0 — no control, 1 — partial control, 2 — full control; ** - 0 - >100 ml, 1 - 50-100 ml, 2 - <50 ml. The differences in indicators before and after the course of treatment are highlighted in background, positive dynamics are in bold, and female patients are in italics.
During the rehabilitation treatment, no adverse events (illnesses, injuries, unplanned surgical interventions, etc.) occurred. There was an increase in spasticity by 1 point at the end of the course in 1 patient from the main group (O8) and 2 patients from the control group (K5, K9).
According to the results of the study of muscle strength, all patients of the main group, in whom muscle strength in the lower extremities was recorded at the beginning of the course, noted its improvement to 2-3 points (patients O5, O8, O10, O12), in 2 more patients who had previously had no muscle activity in the lower extremities, muscle strength appeared up to 2 points (patients O2, O4). Subjectively, patients noted that during the TESCM procedure it was easier for them to exercise on the simulator, stand or perform other doctor’s tasks, they began to feel tension and work of the muscles of the lower extremities, their regulation improved, which significantly influenced the emotional mood of the patients and further motivation to exercise.
In the control group, 1 patient whose muscle strength was recorded at the beginning of the course also showed an increase to 2 points (patient K10).
Comparison of muscle strength scores recorded before and after the course using statistical criteria revealed in the main group a significant increase in the strength of the flexor and extensor muscles of the hip and extensor muscles of the leg ( p
<0.05 by both Wilcoxon and Student's tests).
Statistical analysis of changes in muscle strength in the control group showed a significant increase in the strength of the hip flexor and extensor muscles according to the Wilcoxon test ( p
<0.01) and non-significant according to the Student test (
p
= 0.838). Due to the striking differences between the probabilities calculated using the 2 criteria, no conclusion was made about the statistical significance of the changes in muscle strength in the control group.
When studying sensitivity (see Table 2), in 1 patient of the main group the level of anesthesia dropped by 1 segment. Another 2 patients developed deep sensitivity and muscle-articular sensation in the knee joints (patients O2, O10). Comparison of sensitivity scores recorded before and after the course using statistical tests did not reveal significant differences.
In the control group, no change in sensitivity was noted after the course.
The increase on the ASIA scale in the main group ranged from 1 to 8 points. Patients O2 and O4 were reclassified the level and severity of injury on this scale from B to C (see Table 1).
The increase on the ASIA scale in the control group did not exceed 1 point.
After analyzing the urination diaries kept by the patients, it was revealed that in the main group, 1 out of 3 patients who did not control urination developed the urge to urinate, and he also regained partial control of urination (patient O2). Another 1 patient (O3) also developed partial urinary control. In both cases, the effect of TESCM on urinary function was noted after 3-5 procedures. At the beginning of the course, in 4 patients of the main group (O2, O3, O5, O8), the amount of residual urine was more than 100 ml. At the end of the TESCM course, 2 patients (O3 and O8) had from 50 to 100 ml of urine left after urination, and 1 patient (O2) had less than 50 ml, which corresponds to normal values and allowed him to catheterize less often. In the group as a whole, the differences in the analyzed indicators of urinary function were statistically insignificant.
In the control group, the analyzed indicators of urinary function remained unchanged after the course of treatment.
How does neuromodulation work?
Neuromodulation works either by actively stimulating nerves to produce a natural biological response, or by applying targeted pharmaceutical agents in tiny doses directly to the site of action.
Neurostimulation devices involve the application of electrodes to the brain, spinal cord, or peripheral nerves. These precisely placed electrodes are connected via an extension cable to a pulse generator and power supply that generates the required electrical stimulation. A low voltage electrical current passes from the generator to the nerve and can either suppress pain signals or stimulate nerve impulses where they were previously absent.
In the case of pharmacological agents delivered via implanted pumps, the drug can be administered in smaller doses since it does not have to be metabolized and pass through the body before reaching the target area. Smaller doses—in the range of 1/300th the oral dose—may mean fewer side effects, increased patient comfort, and improved quality of life.
Neuromodulation, whether electrical or magnetic, uses the body's natural biological response by stimulating the activity of nerve cells, which can influence the nerve population by releasing neurotransmitter neurotransmitters, such as dopamine, or other chemical messenger compounds, such as peptide substance P, which can modulate the excitability and firing patterns of neural circuits. More direct electrophysiological effects on neural membranes, such as the mechanism of action of electrical interaction with neural elements, may also be generated. The net effect is the "normalization" of the neural network's function from its excited state. Proposed mechanisms of action for neurostimulation include depolarizing blockade, random (stochastic) normalization of the action potential (nerve impulse), axonal blockade, reduction of nerve impulse keratoses, and suppression of neural network oscillations.2 Although the exact mechanisms of neurostimulation are unknown, empirical effectiveness has led to widespread use of this method in clinical practice.
Existing and new neuromodulation treatments are also used in drug-resistant epilepsy3, chronic headache, and functional therapies ranging from bladder and bowel or respiratory control to improving sensory impairments such as hearing (cochlear implants and auditory brainstem implants) and vision (retinal implants).4 Technical improvements include a trend toward minimally invasive (or non-invasive) systems; as well as smaller and more complex devices that may have automatic feedback control5 and compatibility with magnetic resonance imaging.6
Neuromodulation therapy has been investigated for the treatment of other chronic conditions such as Alzheimer's disease,78 depression, chronic pain,910 and as an adjunctive treatment in stroke recovery.1112
Discussion
The main result of the study is evidence of the effectiveness of the use of tESCM in motor neurorehabilitation. As a result of the course of treatment, a number of patients showed improvement in motor and excretory functions. Thus, after a 2-week course of TESCM in combination with motor therapy and a standard course of restorative treatment, consisting of massage, physical therapy, and mechanical therapy, 6 out of 7 patients showed an increase in muscle strength of the lower extremities, and 3 had an improvement in sensitivity indicators. After completion of the course, in 2 patients the severity of the injury was reduced from ASIA B to ASIA C.
A previous study using tESCS during a short course of motor rehabilitation was aimed at studying the combined effects of spinal cord stimulation and activation of serotonin receptors, so it did not include a group of patients who did not receive stimulation [16]. The group of participants included both patients with spinal cord injury and patients with iatrogenic myelopathy; the course consisted of 16-17 half-hour TESCM procedures against the background of mechanotherapy; half of the patients received buspirone, a serotonin receptor agonist. As a result of the course, on average for the group, a significant increase in muscle strength and sensitivity was obtained. However, it is impossible to associate the results achieved after the rehabilitation course only with tESCM due to the lack of a control group of patients who did not undergo tESCM.
Thus, for the first time in a controlled study, we have shown that tESCS is effective in a short-term course of rehabilitation of motor functions in patients with ASIA B and C severity of spinal cord injury. If we look at studies in which it was demonstrated that a multi-month course of electrical stimulation of the spinal cord leads to restoration of independent walking in patients with severe motor impairments [3-5], it will become obvious that the long process of walking restoration consisted of short courses changing one into another, during which certain rehabilitation tasks were solved. The patients were consistently restored: voluntary movements of the legs in conditions of their external support (when gravity was compensated), the ability to maintain a vertical posture, and independent regulation of stepping movements for the right and left legs separately. This gives reason to believe that 2-3-week courses of tESCM can be used in the conditions of inpatient or outpatient rehabilitation of spinal patients for multi-stage restoration of severe motor disorders, complicating the restored motor skills at each subsequent stage.
In the main group, 1 patient had an increase in spasticity of the lower extremities by 1 point after a course of TESCM. It should be noted that the study cited above [16] also recorded an increase in spasticity by 0.5 points after a course of TESCM. In our study, also in the control group, an increase in spasticity was observed at the end of the course in 2 patients. It seems unlikely that this is due specifically to spinal cord stimulation. Electrical epidural stimulation of the spinal cord is known to be used in some cases to reduce spasticity. In particular, a study was conducted on the possibility of using electrical stimulation of the spinal cord to reduce spasticity in patients with cerebral palsy and spinal cord diseases and injuries [17]. It was shown that stimulation at the Th11 level with a frequency of 100-130 Hz, pulse duration 120-300 ms, amplitude 1.5-4 V over several years led to a decrease in spasticity in both groups. In spinal patients, spasticity decreased from 3.71 ± 0.61 points before stimulator implantation to 2.26 ± 0.56 points after 1–9 years of stimulation. However, the authors of a recently published review [18] of a large number of publications that described cases of reduction in spasticity of the lower extremities due to electrical stimulation of the spinal cord, doubt the validity of the conclusions of these studies due to shortcomings in the methodology of the studies, due to technological limitations of implanted equipment and the lack of full understanding of the mechanisms of spasticity. Thus, the effect of tESCS on spasticity remains to be investigated.
Spinal injuries in a large number of cases cause disturbances in voluntary urination [19, 20]. Normally, the functioning of the bladder is associated with spinal cord segments T11-L2 and S2-S4 [21]. To rehabilitate motor functions of the lower extremities, as a rule, electrical stimulation of the spinal cord is performed at the T12-L4 level [4-6]. In this regard, it is logical to expect that by stimulating the spinal cord to restore motor functions, it is possible to influence excretory functions. In spinal animal studies, electrical stimulation of the spinal cord with parameters used for motor rehabilitation has been shown to affect micturition function [12, 13]. A urodynamic study was performed on spinal patients during the TESCM procedure at the T11 vertebral level [14]. This study found that 30 Hz stimulation reduced detrusor overactivity, detrusor and sphincter dyssynergia, and 1 Hz stimulation initiated bladder emptying. In our study, it was found that a course of tESCS with similar stimulation parameters leads to partial normalization of urinary functions in 3 out of 7 patients. Thus, motor rehabilitation using tESCM may be accompanied by an improvement in excretory functions, and it is obvious that further research is required in order to use tESCM to regulate voluntary urination after spinal cord injury.
4. Implantation and rehabilitation after surgery
Implantation and implementation of all elements of the system for epidural stimulation includes the following steps:
- 1. installation of electrodes;
- 2. test period (5-7 days);
- 3. if testing has a significant positive effect, the electrodes are attached to the interspinal ligaments;
- 4. implantation of the generator subcutaneously;
- 5. connecting system elements with wires;
- 6. programming the generator for a specific clinical situation;
- 7. postoperative rehabilitation and teaching the patient self-help.
Non-invasive magnetic methods
Magnetic neuromodulation techniques are usually non-invasive: no surgery is required to penetrate the magnetic field into the body, since the magnetic permeability of tissue is similar to that of air. In other words: magnetic fields penetrate the body very easily.
These two main methods are closely related because both use changes in magnetic field strength to create electric fields and ionic currents in the body. However, there are differences in approach and equipment. In rTMS (Repetitive transcranial magnetic stimulation (rTMS)), the stimulation has high amplitude (0.5–3 Tesla), low complexity and anatomical specificity is achieved through a highly focal magnetic field. In tPEMF (Transcranial Pulsed Electromagnetic Fields (tPEMF)), the stimulation has low amplitude (0.01–500 millitesla), high complexity and anatomical specificity due to the specific frequency component of the signal.25
Inflammatory processes
Inflammation mainly occurs during myelitis. This syndrome develops over several days or weeks. It is often caused by a viral infection. When myelitis occurs, a person complains of pain in the back and weakness in the muscles, which increases very quickly. In addition, asymmetric paresthesias in the legs are often observed.
Among the main risk factors for the development of myelitis are decreased immunity and hypothermia. The development of inflammation can be triggered by:
- infections;
- injuries;
- radiation therapy;
- toxic poisoning;
- introduction of some vaccines.
This disease is characterized by acute and subacute course. In this case, general signs of inflammation are observed, in particular, such as rapid and constant fatigue, a slight increase in temperature, a feeling of weakness, pain and aching muscles, and headaches.
For diagnosis, cerebrospinal fluid is collected. The nature of the damage and assessment of the nerve structures can be obtained by tomography. Treatment of spinal cord myelitis is selected taking into account the provoking factor. For this purpose, therapeutic methods such as:
- for non-infectious cases - glucocorticosteroids;
- for bacterial - antibiotics;
- muscle relaxants;
- diuretics;
- analgesics;
- vitamins.
If the excretory function is impaired, catheterization of the bladder is performed, and the skin must be lubricated with various ointments to prevent the development of complications.
The rehabilitation program, which includes a specially selected course of exercise therapy and massage sessions, is very important. Full recovery may take several years
Arachnoiditis should also be classified as an inflammatory process. This is a disease in which damage occurs in the arachnoid membrane. The reasons for this may be previous various acute and chronic intoxications, diseases of the nasal sinuses. When carrying out treatment, the source of infection must first be eliminated. For this purpose, antibiotics, metabolic and pathogenetic therapy are prescribed.
Neuromodulation market
The global neuromodulation device industry is expected to grow from $8.4 billion in 2021 to $13.3 billion in 2022, according to market research by Neurotech Reports. The market for implanted spinal stimulators for the treatment of chronic pain was valued at $1.80 billion worldwide in 2014 and is expected to grow to $2.88 billion in 2021. Observers predict double-digit average annual growth rates for the industry as a whole.
This is not surprising given the huge number of potential patients for treatment, as evidenced by the prevalence of the following diseases:
- Epilepsy: 40-50 million patients worldwide.
- Migraine: 26 million in the US alone.
- Spinal cord injuries: 250,000 in the US.
- Parkinson's disease: 1.5 million US residents.
- Urinary incontinence: 13 million adults in the US.
To date, neuromodulation is just beginning to be routinely used as a therapy for appropriately selected patients in these groups. As technology continues to advance and the number of physicians increases, the likelihood of neuromodulation therapy touching people's lives will increase dramatically.
Footnotes
- International Neuromodulation Society, October 1, 2013
- Karas PJ, Mikell CB, Christian E, Liker MA, Sheth SA, Deep brain stimulation: a mechanistic and clinical update, Neurosurgical Focus, November 2013, https://doi.org/10.3171/2013.9.FOCUS13383
- Al-Otaibi FA, Hamani C, Lozano AM, Neuromodulation in epilepsy, Neurosurgery, October 2011, https://doi.org/10.1227/NEU.0b013e31822b30cd
- Krames, Elliot S.; Peckham, P. Hunter; Rezai, Ali R., eds. (2009). Neuromodulation, Vol. 1-2. Academic Press. p. 274. ISBN 9780123742483.
- Wu C, Sharan AD, Neurostimulation for the treatment of epilepsy: a review of current surgical interventions, Neuromodulation, 4 September 2012, doi:10.1111/j.1525-1403.2012.00501.x
- Precision™ Plus Spinal Cord Stimulator System Receives CE Mark Approval as MRI Conditional, Paris, France: Boston Scientific Corporation, August 28, 2012
- Clinical trial number NCT01559220 for “Deep Brain Stimulation for the Treatment of Alzheimer's Disease.” at ClinicalTrials.gov
- Clinical trial number NCT01608061 for “Functional Neuromodulation Ltd.” ADvance DBS-f in Patients With Mild Probable Alzheimer's Disease.” at ClinicalTrials.gov
- Kortekaas R, van Nierop LE, Baas VG, Konopka KH, Harbers M, van der Hoeven JH, et al, A novel magnetic stimulator increases experimental pain tolerance in healthy volunteers – a double-blind sham-controlled crossover study, 2013, doi: 10.1371/journal.pone.0061926
- Shupak NM, Prato FS, Thomas AW, Human exposure to a specific pulsed magnetic field: effects on thermal sensory and pain thresholds, Neuroscience Letters, June 2004, https://doi.org/10.1016/j.neulet.2004.03.069
- Matsumura Y, Hirayama T, Yamamoto T, Comparison between pharmacological evaluation and repetitive transcranial magnetic stimulation-induced analgesia in poststroke pain patients, Neuromodulation, 2013, https://doi.org/10.1111/ner.12019
- Feng WW, Bowden MG, Kautz S, Review of transcranial direct current stimulation in poststroke recovery, Topics in Stroke Rehabilitation, https://doi.org/10.1310/tsr2001-68
- Krames, Elliot S.; Peckham, P. Hunter; Rezai, Ali R., eds. Neuromodulation, Vol. 1-2. Academic Press. pp. 1–1200. ISBN 9780123742483
- Sun FT, Morrell MJ, Wharen RE, Responsive cortical stimulation for the treatment of epilepsy, Neurotherapeutics, January 2008, doi: 10.1016/j.nurt.2007.10.069
- Deer TR, Krames E, Mekhail N, Pope J, Leong M, Stanton-Hicks M, et al., The appropriate use of neurostimulation: new and evolving neurostimulation therapies and applicable treatment for chronic pain and selected disease states. Neuromodulation Appropriateness Consensus Committee, Neuromodulation, August 2014, https://doi.org/10.1111/ner.12204
- Mekhail NA, Cheng J, Narouze S, Kapural L, Mekhail MN, Deer T, Clinical applications of neurostimulation: forty years later, Pain Practice, 2010, https://doi.org/10.1111/j.1533-2500.2009.00341. x
- Bailey, Madeleine, A remote control turns off my spine, The Express. London, UK, May 14, 2013
- Deer TR, Mekhail N, Provenzano D, Pope J, Krames E, Leong M, et al, The appropriate use of neurostimulation of the spinal cord and peripheral nervous system for the treatment of chronic pain and ischemic diseases: the Neuromodulation Appropriateness Consensus Committee, Neuromodulation, August 2014, https://doi.org/10.1111/ner.12208
- Bronstein JM, Tagliati M, Alterman RL, Lozano AM, Volkmann J, Stefani A, et al., Deep brain stimulation for Parkinson disease: an expert consensus and review of key issues, Archives of Neurology, February 2011, doi:10.1001/archneurol .2010.260
- Williams NR, Okun MS, Deep brain stimulation (DBS) at the interface of neurology and psychiatry, The Journal of Clinical Investigation, November 2013, doi: 10.1172/JCI68341
- Medtronic Receives European CE Mark Approval for Deep Brain Stimulation Therapy for Refractory Epilepsy Further Clinical Study Required for Application to US Food and Drug Administration, (Press release), 16 September 2010
- Wilner A, Thalamic Stimulation: New Approach to Treatment of Epilepsy, Medscape Neurology, 22 April 2010
- Lozano AM, Lipsman N, Probing and regulating dysfunctional circuits using deep brain stimulation, Neuron, February 2013, DOI: https://doi.org/10.1016/j.neuron.2013.01.020
- George MS, Nahas Z, Borckardt JJ, Anderson B, Burns C, Kose S, Short EB, Vagus nerve stimulation for the treatment of depression and other neuropsychiatric disorders, Expert Review of Neurotherapeutics, January 2007, https://doi.org/ 10.1586/14737175.7.1.63
- Whissell PD, Persinger MA, Emerging synergisms between drugs and physiologically-patterned weak magnetic fields: implications for neuropharmacology and the human population in the twenty-first century, Current Neuropharmacology, December 2007, DOI: 10.2174/157015907782793603
- Hariz MI, Blomstedt P, Zrinzo L, Deep brain stimulation between 1947 and 1987: the untold story, Neurosurgical Focus, August 2010, https://doi.org/10.3171/2010.4.FOCUS10106
- Wall PD, Melzack R (1996). The challenge of pain (2nd ed.). New York: Penguin Books. pp. 61–69. ISBN 0-14-025670-9.
- Lozano AM, Gildenberg PL, Tasker RR, eds. (2009). Textbook of Stereotactic and Functional Neurosurgery. 1. pp. 16–20.
- Bittar RG, Kar-Purkayastha I, Owen SL, Bear RE, Green A, Wang S, Aziz TZ, Deep brain stimulation for pain relief: a meta-analysis, Journal of Clinical Neuroscience, June 2005, https://doi.org /10.1016/j.jocn.2004.10.005
- Benabid AL, Chabardes S, Torres N, Piallat B, Krack P, Fraix V, Pollak P, Functional neurosurgery for movement disorders: a historical perspective, Neurotherapy: Progress in Restorative Neuroscience and Neurology, Progress in Brain Research, 175. pp. 379–91, 2009, https://doi.org/10.1016/S0079-6123(09)17525-8
- Cookson C, Healthcare: Into the cortex Scientific advances on the brain promise to transform the pharmaceutical industry, Financial Times. London. Retrieved October 11, 2014
- Famm K, Litt B, Tracey KJ, Boyden ES, Slaoui M, Drug discovery: a jump-start for electroceuticals, Nature, April 2013, doi: 10.1038/496159a
- Carroll J, GlaxoSmithKline stakes a pioneering effort to launch 'electroceutical' R&D, Fierce Biotech. Retrieved October 11, 2014
- Birmingham K, Gradinaru V, Anikeeva P, Grill WM, Pikov V, McLaughlin B, et al., Bioelectronic medicines: a research roadmap, Nature Reviews, Drug Discovery (published May 30, 2014), doi:10.1038/nrd435