Alexandra Korostyshevskaya, Andrey Savelov, Irina Prikhodko, Yana Isaeva, Vasily Yarnykh “Science at First Hand” No. 3(88), 2020
Since childhood, we have heard that nerve cells do not recover. And although the question of the possibility of the formation of new neurons in the adult brain is still open, there is already evidence that the process of neurogenesis in humans continues into old age. Any disturbances in the development of nerve cells can lead to serious, sometimes irreversible pathologies. One of these disorders is defects in the protective insulating sheath (myelin) of nerve cell processes, which can form in a person even before birth. They are almost impossible to diagnose using traditional imaging methods.
About the authors
| Alexandra Mikhailovna Korostyshevskaya — Doctor of Medical Sciences, leading researcher at the laboratory of MRI technologies, head of the diagnostic department of the Institute “International Tomography Center” of the Siberian Branch of the Russian Academy of Sciences. Author and co-author of more than 70 scientific papers. |
| Andrey Alexandrovich Savelov — Candidate of Physical and Mathematical Sciences, senior researcher at the laboratory of MRI technologies at the Institute of International Tomography Center of the Siberian Branch of the Russian Academy of Sciences. Author and co-author of more than 90 scientific papers. |
| Irina Yurievna Prikhodko - software engineer at the laboratory of MRI technologies at the Institute of International Tomography Center of the Siberian Branch of the Russian Academy of Sciences. Author and co-author of 3 scientific papers. |
| Yana Olegovna Isaeva - student at the Institute of Medicine and Psychology named after. V. Zelman Novosibirsk State University. |
| Vasily Leonidovich Yarnykh — Candidate of Chemical Sciences, head of the laboratory of the Department of Radiology, professor at the University of Washington (Seattle, USA) and Tomsk State University. Author and co-author of more than 70 scientific papers, including 5 patents. |
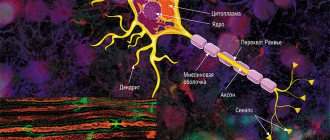
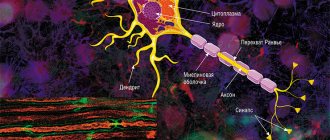
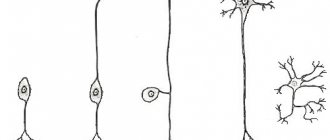
The average human brain contains about 100 billion neurons that receive, store, process and transmit information using electrical and chemical signals. Interaction between a neuron and other nerve cells and organs occurs through short ( dendrites)
) and long (
axon
) processes.
Each axon, like a wire, is covered with an insulating material - the myelin sheath.
, which provides a higher speed of nerve impulses and protects nerve fibers from damage. In addition, this shell has a supporting function, and also, according to the latest data, serves as a kind of “refueling station” for the axon, which needs a large amount of energy.
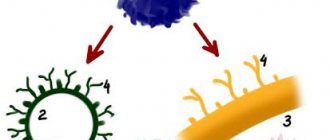
The axon is the main “cable” of a neuron, covered with a myelin sheath. It vaguely resembles a power line with a chain of insulators. The purpose of the shell, which is formed by special service cells (oligodendrocytes or Schwann cells), is to ensure the transmission of electrical impulses without loss and at maximum speed. © Servier Medical Art. Left
- axons of mouse sciatic nerves (
red
), wrapped in Schwann cells (
green
, nuclei -
blue
).
Photo by A. Alvarez-Prats and T. Balla. © Eunice Kennedy Shriver / National Institute of Child Health and Human Development
/ NIH
All damage to the myelin sheath or defects that occur during its formation lead to serious, sometimes incurable diseases. Among them, the most famous is multiple sclerosis.
is a chronic autoimmune disease that primarily affects young people.
Myelin is also destroyed during strokes
, which occur not only in adults (primarily, as is commonly believed, in older people), but also in children, including the unborn.
Intrauterine stroke most often occurs after the 28th week of pregnancy, and in children - a month after birth. Stroke in a fetus leads to the development of brain defects, and in children it can cause cerebral palsy
at an early age.
At the same time, today we judge the “quality” of myelination of the brain of a particular person only by indirect clinical symptoms or magnetic resonance imaging
(MRI), which can usually detect myelin defects at a late, often irreversible stage.
In the brain, the myelin sheath is created by oligodendrocytes, in the peripheral nervous system - by Schwann cells. Each oligodendrocyte forms several “legs” that repeatedly “wrap” around part of an axon ( bottom
).
As a result, one oligodendrocyte is connected to several neurons. © Servier Medical Art. At the top
- oligodedrocytes in culture (
red
, nuclei -
lilac
). © jakeyoung64
Not everyone knows that myelin is many layers of cell membrane, “wound” many times around the axon. Myelin is formed by flat outgrowths of “service” glial cells, in which there is practically no cytoplasm. The myelin sheath is not continuous, but discrete, with gaps (nodes of Ranvier). Therefore, the axon has faster saltatory conduction: the speed of signal transmission along fibers with and without myelin can differ hundreds of times. As for the molecular composition of the “insulator,” it, like all cell membranes, consists primarily of lipids and proteins.
Neural insulation defects
Fetal brain development is a complex process in which rapid changes in the morphology and microstructure of nervous tissue occur. In some areas of the brain, the process of myelin formation begins as early as 18–20 weeks of pregnancy, and continues until approximately the age of ten.
It is myelination disorders that often underlie delays in the physical and mental development of a child, and also cause the formation of a number of neurological and psychiatric pathologies. In addition to diseases such as stroke, delays in fetal brain development with impaired myelination are sometimes observed in multiple pregnancies. At the same time, desynchronization in the development of the brain of twins is quite difficult to assess “by eye”.
But how to identify myelin defects during fetal development? Currently, obstetricians and gynecologists use only biometric indicators (for example, brain size), but these are highly variable and do not provide a complete picture. In pediatrics, even in the presence of obvious functional abnormalities in the child’s brain activity, traditional MRI or neurosonography
(ultrasound examinations of the brain of newborns) often do not show structural abnormalities.
Therefore, the search for accurate quantitative criteria for assessing myelin formation during pregnancy is an urgent task, which also needs to be solved using non-invasive diagnostic methods that have already been tested in obstetrics. Specialists from the Novosibirsk International Tomography Center SB RAS proposed using for these purposes a new method of quantitative neuroimaging, already adapted for prenatal ( prenatal)
) research.
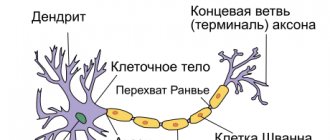
Anatomy of myelin in the structure of a nerve
The main cell of the nervous system is the neuron.
The body of the neuron is called the soma. There is a core inside it. The body of the neuron is surrounded by short processes called dendrites. They are responsible for communication with other neurons. One long process, the axon, extends from the soma. It carries impulses from the neuron to other cells. Most often, at the end it connects with the dendrites of other nerve cells. The entire surface of the axon is covered by the myelin sheath, which is a process of the Schwann cell, devoid of cytoplasm. Essentially, it is several layers of cell membrane wrapped around the axon.
Schwann cells enveloping the axon are separated by nodes of Ranvier, which lack myelin.
On a regular tomograph
Any pathology of the fetal brain that doctors suspect during an ultrasound examination of a pregnant woman is usually an indication for an MRI; Similar studies have been carried out at the ITC SB RAS for more than ten years. MRI results can confirm, clarify, refute, or even change the preliminary diagnosis and, accordingly, pregnancy management tactics.
The fact is that the amount of myelin and the size of individual brain structures in the embryo are so small that any measurements are very complex and time-consuming. In addition, the fetus is constantly moving, which makes it very difficult to obtain high-quality images and reliable quantitative data. Therefore, we need technology that allows us to obtain images quickly and with high resolution even on small objects.
This is exactly what the method for fast mapping of the macromolecular proton fraction
(MPF) is a biophysical parameter that describes the proportion of protons in tissue macromolecules involved in the formation of the MRI signal, whereas the signal source is usually protons contained in water (Yarnykh, 2012; Yarnykh et al., 2015).
The macromolecular proton fraction (MPF) method is based on the magnetization transfer effect, when protons of free water “exchange” magnetization with protons of low-mobility macromolecules, such as proteins. The speed of this process affects the magnitude of the detected MRI signal and depends on the area of interaction between the macromolecular fraction and water
The method is based on a specialized procedure for mathematical processing of MRI images, which makes it possible to isolate signal components associated with the MPF of cell membranes. And in the brain of humans and animals, the main part of them is contained in myelin. MPF maps are reconstructed based on initial data, which can be obtained on almost any clinical tomograph.
To reconstruct the MPF maps, four source images obtained by various traditional MRI methods are used. The correctness of this approach was confirmed by the results of its testing on laboratory animals at Tomsk State University: in mice that were injected with a solution that causes myelin destruction, the results of MPF mapping coincided with the data of histological examination of tissues (Khodanovich et al., 2017).
Ursolic acid restored the myelin sheaths of neurons in mice with sclerosis
Expression of ciliary neurotropic factor in astrocytes upon exposure to ursolic acid
Yuan Zhang et al. / Proceedings of the National Academy of Sciences, 2020
Ursolic acid, when administered orally, stops the development of an analogue of multiple sclerosis in mice, stimulates the maturation of oligodendrocytes and restores the myelin sheath of neurons in the central nervous system. The acid interacts with peroxisome proliferator-activated receptor gamma and, through the transcription factor CREB, triggers the release of ciliary neurotrophic factor, which affects the differentiation of oligodendrocyte precursors. The study was published in the journal Proceedings of the National Academy of Sciences
.
Multiple sclerosis is an autoimmune disease that damages the myelin sheath of neurons throughout the central nervous system. Modern approaches to the treatment of multiple sclerosis make it possible to stop autoimmune reactions and are effective in the early stages of the disease. However, there is no way to restore the myelin sheaths of nerve cells and return them to functionality. There is potential for this - in the foci of demyelination, the precursors of oligodendrocytes (cells that create the membranes of neurons in the central nervous system) accumulate, but they do not develop.
Ursolic acid is found in many plants, including fruit peels. This substance has a whole range of pharmacological properties; it is used as part of medicinal plants for the treatment of Parkinson's disease, rheumatoid arthritis and diabetes. Administration of ursolic acid may also prevent the development of experimental autoimmune encephalomyelitis (EAE, a mouse model of multiple sclerosis).
Scientists from China and the United States, led by Yuan Zhang from Thomas Jefferson University, studied the effect of ursolic acid on mice with EAE. The substance was administered orally, therapy began at the onset of the disease (11 days after the onset of EAE), at its peak (18 days) or in the chronic phase (60 days). At days 30 or 120, mouse spinal cord sections were obtained and immunohistochemically stained for various markers (eg, myelin, axons, oligodendrocytes).
To separate the anti-inflammatory and myelinating effects of ursolic acid, its effect was studied in mice in which the destruction of myelin in the brain was triggered by cuprizone - in this case, inflammatory processes and the presence of immune T cells in the lesion are minimal. In addition, the effect of ursolic acid was tested on astrocyte culture - these cells secrete oligodendrocyte development factors.
All the studies described above were also carried out on animals heterozygous for the peroxisome proliferator-activated receptor gamma (PPARγ) gene. Ursolic acid is an agonist of this receptor, and its activation mitigates inflammatory processes and triggers neuronal protection mechanisms. The authors of the work suggested that interaction with PPARγ is the main mechanism of action of ursolic acid.
The most effective dose of ursolic acid was 25 milligrams per kilogram of body weight per day. The drug significantly reduced inflammation (p < 0.01) and demyelination (p < 0.001) in the brain, as well as the infiltration of leukocytes (Th1 and Th17 cells) into the affected areas (p < 0.001). Acid suppressed EAE, even if treatment was started at the peak of the disease or in its chronic phase.
The severity of the disease on a scale from 0 to 5; The horizontal axis shows the time from stimulation of EAE development. Black - control animals, color - various dosages of ursolic acid, pink - the optimal dose of 25 milligrams per kilogram per day. Black arrows indicate the time of initiation of treatment, A - at the beginning of the development of the disease, B - at the peak
Yuan Zhang et al. / Proceedings of the National Academy of Sciences, 2020
Share
The number of new oligodendrocytes increased, and their precursors decreased, based on which we can conclude that ursolic acid stimulated the differentiation of these cells. Moreover, the therapy increased the number of myelinated neurons and repaired damaged axons and dendrites when treatment was initiated in the chronic phase of the disease.
Spinal cord sections stained with fluorescent dye. Green - oligodendrocyte precursors, pink - young oligodendrocytes, blue - nuclear dye. Top - control, bottom - mice treated with ursulic acid
Yuan Zhang et al. / Proceedings of the National Academy of Sciences, 2020
Share
Myelination (green) of axons (red) of neurons (nuclei colored blue) of the spinal cord. From left to right: before the onset of EAE development, after 60 days; 120 days after disease induction, mice were treated with placebo or ursulic acid for the last 60 days
Yuan Zhang et al. / Proceedings of the National Academy of Sciences, 2020
Share
In animals whose neuronal myelin was destroyed by cuprizone, ursolic acid also caused the maturation of oligodendrocytes and the restoration of membranes. In astrocytes treated with ursolic acid, the amount of ciliary neurotropic factor (p < 0.01), which caused the maturation of oligodendrocytes, was approximately three times higher than in the control.
The effect of ursolic acid was mediated by PPARγ - when these receptors were blocked with antagonists or animals heterozygous for their gene were used, the drug ceased to be effective. The researchers also found that the transcription factor CREB is involved in triggering the release of ciliary neurotropic factor; when it was turned off, astrocytes did not produce the neurotropic factor.
Ursolic acid is currently undergoing clinical trials on patients with tumors and according to preliminary results it is safe. This substance is known to cross the blood-brain barrier and can act directly on neurons in the central nervous system. Ursolic acid is a cheap, safe and easy-to-use substance that could potentially be used in the treatment of chronic multiple sclerosis, but this will require further testing and clinical trials in humans.
There are no drugs that can reverse the destruction caused by multiple sclerosis, but the search for them has been going on for a long time. Several years ago, a possible target for such drugs was found - the expression of the b4 subunit of the G protein stimulates the differentiation of oligodendrocytes and myelination of neurons.
Alisa Bakhareva
Compound
The myelin layer consists of two layers of lipids and three layers of protein. There are much more lipids in it (70-75%):
- phospholipids (up to 50%);
- cholesterol (25%);
- glactocerebroside (20%), etc.
The high fat content causes the myelin sheath to be white, which is why the neurons covered by it are called “white matter.”
Protein layers are thinner than lipid layers. The protein content in myelin is 25-30%:
- proteolipid (35-50%);
- myelin basic protein (30%);
- Wolfgram proteins (20%).
There are simple and complex proteins of nervous tissue.
The role of lipids in the structure of the shell
Lipids play a key role in the structure of the pulp membrane. They are the structural material of nervous tissue and protect the axon from energy loss and ion flows. Lipid molecules have the ability to restore brain tissue after damage. Myelin lipids are responsible for adaptation of the mature nervous system. They act as hormone receptors and communicate between cells.
The role of proteins
Protein molecules are of no small importance in the structure of the myelin layer. They, along with lipids, act as the building material of nervous tissue. Their main task is to transport nutrients into the axon. They also decipher signals entering the nerve cell and speed up reactions in it. Participation in metabolism is an important function of the myelin sheath protein molecules.
Principles of treatment of shell defects
Diseases associated with the destruction of the pulp membrane are very difficult to treat. Therapy is aimed mainly at relieving symptoms and stopping destruction processes. The earlier the disease is diagnosed, the greater the chances of stopping its progression.
Possibilities of myelin restoration
Thanks to timely treatment, the process of myelin restoration can be started. However, the new myelin sheath will not perform its functions as well. In addition, the disease may enter a chronic stage, and the symptoms will persist, only slightly smoothing out. But even minor remyelination can stop the course of the disease and partially restore lost functions.
Modern drugs aimed at myelin regeneration are more effective, but are very expensive.
Therapy
The following drugs and procedures are used to treat diseases caused by the destruction of the myelin sheath:
- beta interferons (stop the course of the disease, reduce the risk of relapses and disability);
- immunomodulators (affect the activity of the immune system);
- muscle relaxants (help restore motor functions);
- nootropics (restore conductive activity);
- anti-inflammatory (relieve the inflammatory process that caused the destruction of myelin);
- neuroprotectors (prevent damage to brain neurons);
- painkillers and anticonvulsants;
- vitamins and antidepressants;
- cerebrospinal fluid filtration (a procedure aimed at cleansing cerebrospinal fluid).
Functions
The main functions of the myelin sheath are:
- axon isolation;
- acceleration of impulse conduction;
- energy savings by maintaining ion flows;
- nerve fiber support;
- axon nutrition.
How pulses work
Nerve cells are isolated due to their membrane, but are still interconnected. The areas where cells touch are called synapses. It is where the axon of one cell and the soma or dendrite of another meet.
An electrical impulse can be transmitted within a single cell or from neuron to neuron. This is a complex electrochemical process that is based on the movement of ions through the membrane of the nerve cell.
In a calm state, only potassium ions enter the neuron, and sodium ions remain outside. At the moment of excitement they begin to change places. The axon is positively charged from within. Then sodium stops flowing through the membrane, but the outflow of potassium does not stop.
The change in voltage due to the movement of potassium and sodium ions is called the "action potential". It spreads slowly, but the myelin sheath that envelops the axon speeds up this process by preventing the outflow and influx of potassium and sodium ions from the axon body.
Passing through the node of Ranvier, the impulse jumps from one part of the axon to another, which allows it to move faster.
Once the action potential crosses the break in the myelin, the impulse stops and the resting state returns.
This method of energy transfer is characteristic of the central nervous system. As for the autonomic nervous system, it often contains axons covered with little or no myelin. There are no jumps between Schwann cells, and the impulse travels much more slowly.