Parkinsonism is a syndrome characterized by a combination of hypokinesia with at least 1 symptom: resting tremor, rigidity and postural instability. The most common form of parkinsonism syndrome is idiopathic parkinsonism, or Parkinson's disease. Neurologists also distinguish secondary parkinsonism, the development of which is associated with the influence of a certain etiological factor. Parkinsonism syndrome may be one of the main or additional manifestations of other degenerative diseases affecting the extrapyramidal system.
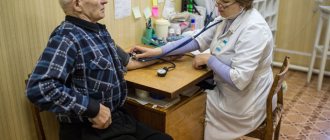
Neurologists at the Yusupov Hospital carry out differential diagnosis of Parkinson's disease and parkinsonism syndromes using innovative research methods. Patients are examined using the latest, high-tech equipment. Differential diagnosis of Parkinson's disease and parkinsonism syndrome is quite complex. Primary Parkinson's disease can be hidden even under the diagnosis of "osteochondrosis of the spine with radicular syndrome", and, conversely, essential tremor is interpreted as Parkinson's disease.
Diagnosis and treatment of early stage Parkinson's disease
O.S. Levin1,2, A.V. Rosinskaya3
1Russian Medical Academy of Postgraduate Education; 2Center for Extrapyramidal Diseases (Moscow); 3Room of extrapyramidal disorders of Primorsky Regional Clinical Hospital No. 1 (Vladivostok)
Parkinson's disease (PD) is a progressive neurodegenerative disease characterized by the combination of hypokinesia with muscle rigidity and/or resting tremor, as well as later developing postural instability and a wide range of non-motor disorders, including autonomic, mental, dyssomnic and sensory symptoms. The formation of neurotoxic aggregates of the small presynaptic protein alpha-synuclein (the main component of Lewy bodies), accompanied by the death of pigmented neurons in the ventrolateral parts of the substantia nigra pars compacta, is considered to be the main link in the pathogenesis of PD. However, in recent years it has been established that only the main motor symptoms of parkinsonism correlate with damage to the substantia nigra; at the same time, the degenerative process in PD also involves other groups of neurons in various areas of the brain, as well as in the peripheral nervous system, which underlies numerous non-motor manifestations of the disease [1, 3].
Types of secondary, symptomatic parkinsonism
The following types of secondary, symptomatic parkinsonism are distinguished:
- drug. It can be provoked by neuroleptics, lithium preparations, rauwolfia preparations, serotonin reuptake inhibitors, α-methyldopa, calcium antagonists;
- toxic. Develops as a result of the toxic effect of the organic compound MPTP, manganese, carbon monoxide, carbon disulfide, cyanide, methanol, organophosphorus compounds, and petroleum products;
- with volumetric processes of the brain (malignant and benign tumor, syphilitic gumma, tuberculoma in the area of the basal ganglia or substantia nigra);
- vascular. Develops with infarctions in the basal ganglia, thalamus, frontal lobes or midbrain, as well as diffuse ischemic lesions of the white matter (Binswanger's disease);
- posthypoxic, caused by bilateral necrosis of the basal ganglia, which are very sensitive to ischemia and hypoxia due to the lack of collateral blood flow;
- encephalitic (with neuroborreliosis, AIDS, progressive consequences of Economo's encephalitis lethargica);
- traumatic (boxer encephalopathy, consequences of severe traumatic brain injuries);
- for hydrocephalus (for obstructive or normotensive internal or mixed hydrocephalus).
Parkinsonism occurs in the following degenerative diseases with damage to the extrapyramidal system (atypical parkinsonism, “parkinsonism-plus”), this is the most severe group of diseases, more malignant than Parkinson’s disease:
- multisystem atrophy, which includes three types: striatonigral degeneration, olivopontocerebellar degeneration, Schry-Diger syndrome;
- multiple system atrophies;
- progressive supranuclear palsy (Steele-Richardson-Olszewski disease);
- corticobasal degeneration;
- diffuse Lewy body disease;
- Alzheimer's disease;
- parkinsonism-ALS-dementia;
- Creutzfeldt-Jakob disease, etc.;
Parkinsonism can be classified as a separate group in storage diseases due to genetically determined dysmetabolic disorders:
- Fahr's disease (calcium builds up in the brain);
- Wilson's disease (copper accumulation).
Epidemiology
According to a continuous population study, the prevalence of PD in Russia is 139 cases per 100,000 population, the incidence of PD is 16 cases per 100,000 population per year. With increasing age, the risk of PD increases, and the detection rate of PD among people over 65 years of age is already about 1%. Most cases of the disease occur between the ages of 60 and 70 years. However, in 15% of cases, PD debuts before the age of 45 years.
Based on the available data and the age-sex structure of the Russian population, we can roughly estimate the total number of patients with PD in our country at 210 thousand, with the disease occurring in approximately 20 thousand patients each year. Approximate calculations show that at least a quarter of patients (that is, more than 50 thousand) end up outside the scope of medical care, and most of these are patients with an early stage of the disease [3].
Early onset parkinsonism
Early onset parkinsonism. For almost two centuries that have passed since the description of idiopathic parkinsonism, this problem has always remained the focus of active interest on the part of specialists from various fields of neuroscience. This is due to the high prevalence of the disease, as well as the close relationship between the successes of fundamental biomedical disciplines (from pathoneuromorphology to biochemistry and molecular genetics) and progress in the development of new methods for diagnosing and treating parkinsonism, and understanding the subtle mechanisms of its development. Currently, all cases of parkinsonism can be divided into:
- primary (idiopathic) parkinsonism . It includes Parkinson's disease and a special genetically determined form of early parkinsonism - the so-called juvenile parkinsonism;
- secondary parkinsonism . This syndrome develops as one of the clinical manifestations (complications) of a number of independent diseases and lesions of the central nervous system. The most well-known variants of secondary parkinsonism are vascular, toxic (including medicinal), post-traumatic, etc.;
- parkinsonism in multisystem neurodegenerative diseases (so-called parkinsonism “plus”). Among the diseases that naturally manifest themselves as parkinsonism plus syndromes, we should primarily mention progressive supranuclear palsy, multiple system atrophy, dementia with Lewy bodies, corticobasal degeneration;
- parkinsonism in hereditary diseases of the central nervous system . This is a very large group of diseases of very different origins, which includes hepatolenticular degeneration, Hallervorden-Spatz disease, dopasensitive dystonia, rigid form of Huntington's disease, a number of forms of lipidoses, mitochondrial encephalopathies, etc.
Over 70% of all cases of parkinsonism syndrome in the population account for primary parkinsonism and, first of all, Parkinson's disease. The disease occurs everywhere, its frequency varies from 150 to 300 per 100,000 population, increasing sharply with age. In the age group over 60 years, this disease occurs in 1-2% of people, making Parkinson's disease the second most common neurodegenerative disease after Alzheimer's disease.
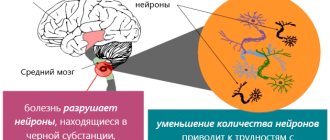
In recent years, it has been established that with the gradual, long-term development of Parkinson's disease, the earliest and most subtle changes (Lewy bodies and Lewy neurites) are found in the projection neurons of the caudal nuclei of the brain stem, and only then the process naturally spreads to the overlying parts of the brain. Nevertheless, the main symptoms of the disease (rest tremor, bradykinesia, muscle rigidity, postural disturbances, etc.) are the result of the death of large dopamine-producing neurons in the compact part of the substantia nigra of the midbrain, disruption of nigrostriatal and other connections, and insufficiency of dopaminergic transmission in the basal ganglia. Thanks to the mechanisms of neuroplasticity, symptoms appear only in the case of the death of >70% of the pigment cells of the substantia nigra, which corresponds to a decrease in dopamine levels by 80-85%. Normally, as a result of the natural aging processes of the body, starting from the 5th decade of life, from 4.7 to 6% of the cells of the substantia nigra die in each decade, which determines the age-dependent nature of Parkinson’s disease.
At the same time, in some cases, parkinsonism can develop at a young age. This aspect of the problem has always received much less attention and, moreover, the established long-term practice almost guaranteed to exclude the diagnosis of Parkinson's disease when symptoms manifest before 40 years of age. Today, our approaches and clinical practice itself have changed dramatically, so that the appearance of various forms of primary parkinsonism in young people has become the everyday reality of a neurologist.
It is interesting that early cases of parkinsonism are characterized by certain features of sensitivity to certain groups of antiparkinsonian drugs, indicating the existence of differences in the pathogenesis of these forms compared with “classical” Parkinson’s disease.
Early-onset parkinsonism (early-onset parkinsonism) is usually called cases of primary parkinsonism that developed before the age of 45 years. Within this age group, an independent subgroup of people with juvenile (juvenile) parkinsonism is often identified, in whom primary parkinsonism manifested itself in the first two decades of life (according to some authors, up to 25 years). The overwhelming majority of cases of juvenile parkinsonism are associated with recessive mutations of the recently discovered parkin genes, DJ.1 and PINK1, the products of which control the processing of neuronal proteins and the characteristics of the oxidative metabolism of nigral neurons; these cases are referred to as autosomal recessive juvenile parkinsonism.
Finally, at a young age, a variety of Parkinson's syndromes may manifest, caused by certain specific toxins, systemic metabolic disorders and other causes. Among toxic Parkinson's syndromes, we note manganese parkinsonism, which has become a serious problem in recent years due to the use of surrogate manganese-containing narcotic compounds by predominantly young people, as well as parkinsonism due to the use of synthetic heroin.
Thus, early parkinsonism is extremely heterogeneous. Further in this review we will talk about primary parkinsonism in young people.
The last few decades have been characterized by a certain trend toward “rejuvenation” of parkinsonism. Among the reasons for this are:
- implementation of the effect of a number of genetic factors;
- the growing exposure of the population of developed countries to adverse environmental impacts;
- improved diagnosis of the disease in its initial stages, associated with general technological progress in clinical medicine.
All these factors deserve detailed consideration.
The possibility of developing “true” Parkinson’s disease at a young age is confirmed by the discovery on the section of typical “Lewy pathology” (including the use of immunohistochemical staining for a-synuclein) in the corresponding parts of the brain in individuals in whom the symptoms of asymmetric levodopasensitive parkinsonism first appeared on 4-5th decade of life. These cases of early Parkinson's disease can be either sporadic or familial. The early onset of Parkinson's disease is usually associated primarily with genetic factors, many of which have been discovered and fairly well studied thanks to the intensive progress of recent years in the field of molecular genetics. An association of Parkinson's disease with a number of polymorphisms in the genes for xenobiotic detoxification, the cell's antioxidant defense system, dopamine transport and metabolism, lipid metabolism, and the mitochondrial cycle has been shown. The susceptibility genes for Parkinson's disease are presented below:
Genes of cellular detoxification and antioxidant defense systems
- paraoxonase-1
- ubiquitin C-terminal hydrolase L1
- cytochrome P450 (CYP2D6)
- N-acetyltransferase-2
- glutatine transferase enzyme family heme oxygenase-1
- enzymes of the a-ketoglutarate dehydrogenase complex
- superoxide dismutase
Genes for dopamine transport and metabolism
- monoamine oxidases A and B
- catechol-O-methyltransferase
- tyrosine hydroxylase
- dopamine transporters
- dopamine receptors D2, D3, D4 and D5
Mitochondrial genome
- tRNAGlu
- mitochondrial DNA (selected polymorphisms)
- complex I of the electronic respiratory chain
Other genes
- NO synthases (nNOS, iNOS)
- apolipoprotein E
- neurotrophic factors
Carriage of unfavorable allelic variants of these genes significantly increases the risk of the disease, i.e. forms a genetic predisposition to Parkinson's disease, which can be assessed quantitatively - for example, by standard calculation of the odds ratio (OR indicator). We and other researchers have found that the identified associations (as well as familial clustering of the disease) are significantly higher in the group of young parkinsonism. Moreover, the combination of several unfavorable polymorphisms increases susceptibility to Parkinson's disease and leads to earlier manifestation of symptoms (additive effect of “risk genes”). Thus, the ratio of genetic and environmental factors in the development of Parkinson’s disease is not the same in different age groups of patients: in the young group of patients the proportion of the genetic component is most significant, while in older patients the role of genetics becomes less clear, and environmental and other factors come to the fore .
Speaking about the genetic load, we must not forget about the accumulation of mitochondrial DNA mutations, the rate of which in modern conditions can be higher, and the consequences in the form of disruption of the energy supply of neurons can be more dramatic and earlier. Let us note in this regard that more than half of people with Parkinson's disease have a decrease in the activity of mitochondrial complex I in various tissues, including the brain.
A well-known modifier of the age of onset of Parkinson's disease and a number of other neurodegenerative diseases is the genetic polymorphism of apolipoprotein E (apoE), a protein related to the repair of cell membranes and the mobilization of “building” cholesterol. Both in our work and in a number of works by foreign authors, it was found that the presence of the apoE-e4 allele contributes to an earlier onset of Parkinson’s disease.
This effect is most pronounced in cases of homozygosity for this variant of apoE (genotype e4/e4): in such cases, according to our observations, the age of onset of the disease was 35-37 years.
According to various sources, from 5 to 10% of all cases of Parkinson's disease are not multifactorial, but monogenic in nature, representing diseases with autosomal dominant inheritance. They are caused by the transmission over generations of pathogenetically significant mutations in the genes a-synuclein, UCH.L1, LRRK2 and some others. The last, very recently discovered gene (LRRK2) is of particular importance, since it can cause up to 1% of all cases of Parkinson's disease in the population - including (since the gene penetrance does not exceed 70%) in the absence of a clear family history.
It can be concluded that in the general series of cases of “ordinary” sporadic Parkinson’s disease, certain hereditary forms are often “masked,” which emphasizes the heterogeneity of this pathology. Hereditary cases of Parkinson's disease are characterized by a relatively early onset of symptoms: for example, in a series of our cases, the disease in carriers of a mutation in the LRRK2 gene could manifest itself as early as 39 years of age; earlier variants of LRRK2 forms of Parkinson's disease have also been described.
Among the factors contributing to the development of Parkinson's disease, great importance is attached to the exposure of the population of developed countries to adverse environmental influences and, first of all, to potential neurotoxins. The most likely candidates for the role of exogenous “causative” toxins in Parkinson’s disease are some pesticides: it has been shown that in vitro, pesticides can provoke conformational changes in the a-synuclein molecule (this is a key stage in the pathogenesis of Parkinson’s disease) and accelerate the formation of pathological inclusions in neurons. Chronic systemic exposure to pesticides experimentally reproduces the clinical characteristics of Parkinson's disease. Epidemiological studies support these conclusions. Thus, the prevalence of Parkinson's disease in rural populations among farmers turned out to be almost 1.4 times higher compared to the urban population, and the risk of Parkinson's disease among plantation workers was 1.5-2 times higher than the general population, which may confirm the role in the development of the disease pesticides and other agents, exposure to which is, by definition, higher among agricultural workers. It is assumed that aggressive agrochemical production and the corresponding environmental conditions characteristic of several regions of the world may be a serious factor contributing to the overall increase in the incidence of Parkinson's disease and, in particularly unfavorable scenarios, the accumulation of earlier cases of the disease in certain subpopulations. The likelihood of the manifestation of early parkinsonism in such a situation especially increases in individuals who are carriers of unfavorable allelic variants of “predisposing” genes responsible for the processes of cellular detoxification in the body (see above). Interestingly, the associations of Parkinson's disease with allelic variants of detoxicant genes (CYP2D6, GSTP1, etc.) are especially significant in the group of individuals who had contact with pesticides. This example clearly illustrates the interaction of genetic and environmental factors in the development of Parkinson's disease.
A certain role in increasing the proportion of young patients with Parkinson's disease belongs to improving diagnostic methods and improving recognition of the earliest cases of the disease (and even “pre-disease”). Let us note, firstly, the introduction into practice of the concept of a risk group, which includes, in particular, the immediate relatives of patients who have a several times higher likelihood of developing Parkinson’s disease compared to the general population. It is in this group of people who are well aware of the existing family history that careful monitoring becomes possible, aimed at identifying the earliest possible disorders in the motor sphere. Secondly, the development of the latest neuroimaging methods (CT and MRI, SPECT, PET), which allow for a more accurate differential diagnosis and objectification of subtle disorders of dopamine metabolism in the basal ganglia, was of particular importance. Finally, one cannot fail to note the role of the unified criteria for the clinical diagnosis of Parkinson's disease developed by the international community, which have made it possible to improve and standardize approaches to the early detection and diagnosis of this disease.
In the figure above, on the left side of the spectrum, a special genetically determined disease is presented - autosomal recessive juvenile parkinsonism (AR.JP), which causes a significant proportion of cases of “young” parkinsonism and is characterized by a number of unique clinical and morphological manifestations. This disease occurs in almost all studied populations of the world.
Of primary importance in the development of AR-JP is a gene localized on chromosome 6q and encoding a new protein with ubiquitin ligase function—parkin. It has been shown that Parkin is an important part of the cellular defense system and, in particular, is directly involved in the degradation of a-synuclein, a classic protein marker of Parkinson's disease in the composition of characteristic intraneuronal inclusions (Lewy bodies).
The morphological picture of AR.JP is characterized by neuronal death and gliosis in the substantia nigra pars compacta and locus coeruleus, differing from “classical” Parkinson’s disease by the absence of Lewy bodies in degenerating neurons.
The onset of AR-JP symptoms most often occurs in the 2nd-3rd decade of life; the first manifestation of the disease may be a gradually developing parkinsonism syndrome or foot dystonia. Parkinsonism syndrome in the advanced stage of AR.JP is typically characterized by a combination of bradykinesia, muscle rigidity, postural disturbances with pyramidal symptoms, as well as the frequent absence of the hemiparkinsonism stage.
A peculiarity of tremor in AR-JP is its statokinetic nature, which can be combined with a typical Parkinsonian resting tremor. Manifestations of dystonia in AR-JP, having appeared in a number of patients at the onset of the disease, can persist for many years. An important feature of the disease, which has significant differential diagnostic significance, is the very early appearance of various and often structurally complex levodopa-induced dyskinesias, which can occur even when taking extremely low doses of the drug (30-70 mg of levodopa). Another distinctive feature of AR-JP, noted in most patients, is the presence of fluctuations in the severity of symptoms of parkinsonism and dystonia throughout the day: the best condition is observed in the morning or after a daytime nap, and in the evening the severity of clinical manifestations increases. This brings the AR-JP clinic closer to the manifestations of dophasensitive torsion dystonia.
Homozygous mutations of the Parkin gene in primary parkinsonism with the onset of the disease before 20 years of age are detected in more than 70% of familial and 15% of sporadic cases. Homozygous mutations in the PINK1 and DJ-1 genes are detected much less frequently in AR-JP. Rarely, the onset of the disease at a later age (up to the 6th decade of life!) has been described in homozygous carriers of parkin gene mutations, and such cases may be indistinguishable from “classical” Parkinson’s disease [30]. This demonstrates a certain convention of the term “juvenile parkinsonism” in relation to parkin-associated forms of pathology (the term “parkinopathies”, which is increasingly recognized, seems more adequate to designate these syndromes).
More recently, there has been evidence to suggest that heterozygous carriage of a mutation in the parkin gene is sometimes sufficient for the development of a dominant form of parkinsonism - most likely as a result of a fall below the critical “threshold” of the ligase activity of the protein product of the gene (haploinsufficiency mechanism). An intravital PET study showed a clear decrease in fluorodopa uptake in the striatum in individuals with a mutant and normal parkin allele, which is clear neuroimaging evidence of dopaminergic dysfunction in heterozygotes.
Taking these data into account, we searched for structural rearrangements in the parkin gene in 107 patients with early parkinsonism (age of onset up to 45 years). At the same time, parkin mutations were detected in 13.1% of patients (14 patients), including heterozygous deletions and duplications in 12 out of 14 cases. Similar data were obtained by other researchers.
Thus, Parkin haploinsufficiency may indeed determine the death of dopamine neurons and serve as a significant risk factor for primary parkinsonism at a young age. We emphasize that the disease in heterozygous carriers of parkin mutations differs from AR-JP in its genetic and clinical characteristics, being an independent and still poorly studied variant of parkin-associated parkinsonism.
The early onset of primary parkinsonism places increased demands on the rationalization of prescribed therapy, since such therapy should be oriented for the long term, ideally for decades to come.
Most researchers prefer to start the treatment of early parkinsonism with dopamine receptor agonists (DRAs). The feasibility of their use in this group of patients is due to the better tolerability of ADR in young patients compared to elderly patients, as well as the need to take into account the possibility of rapid onset of severe dyskinesias in young patients when levodopa is prescribed (this is most typical for various variants of juvenile parkinsonism). In addition, it is for young patients in the initial stage of the disease that the neuroprotective effect provided by drugs from the ADR group (confirmed in experiments and in some clinical neuroimaging studies) is important, which allows to prolong the course of the disease to a certain extent. ADR-mediated neuroprotection is associated with a decrease in the synaptic circulation of dopamine, stimulation of D1 receptors, synthesis of proteins with antioxidant properties, stimulation of autotrophic activity of neurons, and a decrease in the secretion of the excitotoxin glutamate.
If necessary, in young patients, ADR can be combined with MAO-B inhibitors, as well as amantadines (midantan, PC-Merz, etc.). The last group of drugs is quite promising for the treatment of young cases of parkinsonism, since available data indicate that they have properties as antagonists of NMDA glutamate receptors; Thus, amantadines are able to realize their putative neuroprotective effect at the level of the “excitotoxic cascade”.
In the young age group, to combat tremor (a symptom of Parkinson's disease that is very resistant to treatment), central anticholinergic drugs can be more freely prescribed, which are usually not recommended in older people due to the large number of general somatic contraindications, the risk of increasing cognitive impairment and the risk of developing psychotic conditions in the background. brain atrophy. It should be remembered that in young patients, central anticholinergics are prescribed in the minimum possible dosage, and the total duration of continuous treatment with these drugs should not exceed 3-5 years.
At a certain stage of the disease, when movement disorders increase, it becomes necessary to take levodopa drugs. According to modern concepts, the “danger” of levodopa in terms of its adverse effect on the course of Parkinson’s disease remains unproven, and untimely (excessively delayed) administration of levodopa can neutralize the existing therapeutic potential of replacement therapy and, thereby, have a negative impact on the prognosis of the disease and quality of life . Avoiding unfounded “levodopophobia”, it should be remembered that young patients require especially careful “titration” of single and daily doses of levodopa, minimizing the manifestations of dyskinesias that are often very painful for the patient.
Control of motor complications of levodopa therapy involves the prescription of a variety of pathogenetic and symptomatic correctors (ADRs, COMT inhibitors and the new combined levodopa drug Stalevo - see below, benzodiazepines, etc.).
According to our experience, in at least half of patients with juvenile parkinsonism, the use of levodopa-containing drugs was impossible without their combination with ADR. Taking into account life expectancy and the need to delay motor fluctuations as much as possible, in young patients it seems strategically justified to begin levodopa therapy with its long-acting forms.
In recent years, a number of new areas of treatment for parkinsonism have emerged, related both to functional neurosurgery (high-frequency electrical stimulation of the basal ganglia), and to original methods of transdermal delivery of antiparkinsonian drugs and minimally invasive surgery technologies. The so-called concept of constant dopaminergic stimulation deserves attention, which can be implemented, in particular, through dosed administration of levodopa through a constant duodenal pump; this makes it possible to effectively stop severe motor fluctuations in patients in the advanced stage of the disease. Another approach to prolong and “physiologize” the effect of levodopa is elegantly implemented in a new and extremely promising dosage form, which is a combination of levodopa, carbidopa and entacapone (Stalevo). The current experience shows the high effectiveness of Stalevo in reducing the severity of motor fluctuations and reducing the risk of developing dyskinesias both in the clinic and in the experiment, which is associated with the improved pharmacokinetics of levodopa (simultaneous inhibition of peripheral dopa decarboxylase and catechol-O-methyltransferase). The potential protective effect of Stalevo with early administration of the drug is discussed.
The use of the above and a number of other new technologies for various forms of primary parkinsonism is indicated, first of all, in young patients who have fewer contraindications and unfavorable prognostic factors. This significantly expands the available possibilities for effective assistance to people of working age suffering from early parkinsonism, which has not only a medical, but also an undoubted socio-economic effect.
Illarioshkin S.N. Atmosphere. Nervous diseases, 3, 2006
Diagnostics
The diagnosis of PD is carried out in 2 stages. At the first (syndromic) stage, parkinsonism syndrome must be distinguished from other conditions that mimic it (Table 1).
Table 1. Conditions requiring differential diagnosis with parkinsonism.
| If there is tremor | In the absence of tremor |
| Increased physiological tremor Essential tremor Dystonic tremor Hepatolenticular degeneration | Apathetic-abulic syndrome Depression Frontal dysbasia Humeral periarthropathy Hypothyroidism Cervical osteochondrosis Dementia with the phenomenon of paratonia (continuity) Catatonia |
Identifying signs of hypokinesia is key in differential diagnosis. Initial symptoms of hypokinesia may be characterized by difficulty writing, pressing buttons on a remote control, brushing teeth, typing on a keyboard, removing small objects such as coins from a bag or pocket, putting on slippers, etc. Sometimes, already at an early stage, weakness and lag of one of the legs when walking appears with a change in the usual gait pattern. Characterized by a weakening of friendly arm movements when walking (acheirokinesis), impaired recharging of the watch (“Rolex symptom”). A weakened voice, slowness, weakening of intonation, or unclear speech may be noticed (especially when pronouncing morphologically complex words quickly). When examined to identify hypokinesia, the patient is asked to perform certain movements for about 20 seconds at the fastest pace and with maximum amplitude. In this case, the doctor should pay attention to the slow initiation of movement, asymmetry of movements, but most importantly - to a special form of exhaustion of movements (decrement), which, as they are repeated, become increasingly slower, decrease in amplitude, and require more and more effort from the patient. The phenomenon of exhaustion can be detected in all movements assessed, but is sometimes noted only in one of the tests. It should be borne in mind that the slowness and awkwardness of movements characteristic of patients with parkinsonism at an early stage can be confused with manifestations of pyramidal and cerebellar insufficiency, as well as severe depression, however, these conditions are not characterized by a decrement of movements as they are repeated. It should be borne in mind that hypokinesia can be difficult to detect against the background of severe tremor in a limb, however, even in this case it is important not to miss a diagnostically significant phenomenon: with parkinsonism, after performing a test for hypokinesia, the patient often holds his hand in a fixed, tense position and is not able to quickly relax.
Muscle rigidity is manifested by stable (as opposed to spasticity) resistance to passive movements in the wrist, elbow, shoulder, knee joints, as well as in the neck, and subjectively by stiffness and unpleasant painful sensations in the limbs. In some patients, when checking their tone, the “gear wheel” phenomenon is revealed. Rigidity should be distinguished from the phenomenon of resistance (gegenhalten), characteristic of patients with dementia and damage to the frontal lobes. Counter-containment changes rapidly depending on the direction and speed of passive movement.
Slow (3–4 Hz) resting tremor in one arm or leg is one of the common initial manifestations of parkinsonism. The presence of a classic resting tremor of the “rolling pills” or “counting coins” type is most characteristic of PD. To identify latent tremor, the patient is asked to make movements with the other hand, walk around, and perform a distraction task (for example, subtracting from 100 by 7). To identify tremors in the leg, you need to examine the patient in a sitting or lying position. However, in the absence of hypokinesia, rest tremor does not allow diagnosing either parkinsonism or PD. It should be taken into account that, on the one hand, essential and dystonic tremor can be observed at rest, on the other hand, postural and kinetic tremor is often observed in PD.
The initial manifestation of PD, especially in young people, may be foot dystonia, which appears or worsens when walking, and much less often - dystonia of other localization.
Early non-motor disorders. Starting from the earliest (prodromal) stage of the disease, the patient may be bothered by emotional depression, increased irritability, fatigue or a feeling of constant fatigue, as well as autonomic disorders such as sweating disorders (“defective thermostat”), for example, profuse sweating in cold weather, and also a tendency to constipation, frequent and/or imperative urination, increased drooling at night (the “wet pillow” symptom), erectile dysfunction. Hypoosmia often occurs already in the premotor stage of PD, but rarely attracts the attention of the patient himself, and a formalized study is necessary to identify it (using special techniques, for example, the University of Pennsylvania Olfactory Test - UPSIT). Identifying signs of rapid eye movement sleep behavior disorder syndrome (anxious dreams, vocalizations, sleep-talking, movements reflecting the content of dreams), which may precede other manifestations of the disease for many years, may be of important diagnostic importance. These non-motor manifestations may improve the accuracy of diagnosis based on early motor symptoms of the disease.
The debut manifestations of PD are also chronic pain syndromes, most often in the back and scapulohumeral region, associated with increased muscle tone, limited mobility and postural disorders.
Already at an early stage, signs of mild cognitive impairment may be detected, in particular instability of attention and slowness of thinking, difficulty finding words (the “tip of the tongue” phenomenon).
"Red flags". The second stage - the stage of nosological diagnosis - comes down to the differential diagnosis of PD with other nosological forms of parkinsonism. It requires a clinical assessment of the history and neurological examination findings. It is important to clarify the drug history. Drugs such as metoclopramide, sodium valproate, cinnarizine, amiodarone can cause drug-induced parkinsonism. Discontinuation of the drug that triggered the development of parkinsonism may not lead to immediate regression of symptoms. Sometimes, after taking the drug and a short-term improvement, the condition worsens again, which indicates a latently developing degenerative process that was “unmasked” by the side effects of the drugs.
A neurological examination may reveal symptoms that are atypical for PD, requiring the exclusion of other diseases that cause parkinsonism. Among them are: symmetry, rapid progression of symptoms with early loss of the ability to move within 5 years, early development of postural instability with falls, lack of a persistent positive effect of adequate doses of levodopa, early development of autonomic failure, rapid onset of dementia (within 1- year), limited mobility of the eyeballs (especially paresis of downward gaze), early development of severe pseudobulbar syndromes, axial dystonia, pyramidal and cerebellar signs, the presence of focal disorders of cortical functions.
Causes
The main cause of the disease is the death of neurons in the area of the brain that is responsible for the coordinated action of muscles and muscle tone. Neurologists have also noted a lack of dopamine in this area of the brain, which causes symptoms such as tremors and muscle stiffness. But there are quite a lot of risk factors or prerequisites. They do not necessarily directly lead to the development of the disease, but they significantly increase its likelihood.
Only a neurologist can identify the exact cause after a full examination.
Additional research methods
At the moment, there are no laboratory or instrumental research methods that would be mandatory for every patient with suspected PD. In recent years, patients with PD have often undergone CT or MRI of the brain, but most often this is not necessary, and in most cases the diagnosis can be made based on clinical findings. However, if the clinical picture in a patient with parkinsonism syndrome deviates from the classic variant characteristic of PD, in particular, there is no typical response to dopaminergic drugs, then neuroimaging is necessary.
When the onset of the disease is before 50 years of age, it is important to exclude hepatolenticular degeneration, which may be indicated by a corneal Kayser-Fleischer ring, low ceruloplasmin levels, increased signal intensity from the basal ganglia and cerebellum on T2-weighted MRI images, and increased urinary copper excretion.
Transcranial sonography of the deep structures of the brain may also have diagnostic significance, revealing in PD hyperechoic changes in the projection of the substantia nigra, associated with the accumulation of iron and established in 92% of cases of clinically probable PD, but its results can only be interpreted in a clinical context.
Of the practically important, but not yet available in our country, diagnostic methods include positron emission tomography (PET) and single-photon emission computed tomography (SPECT), which make it possible to study synaptic transmission at all levels, as well as monitor the pathological process. When detecting a decrease in the accumulation of F18-fluorodopa with PET and β-CIT with SPECT in the striatum, we can talk about the involvement of presynaptic nigrostriatal terminals in the pathological process (primary parkinsonism). Determination of decreased accumulation of 11C-raclopride (D2 receptor ligand) in PET will indicate a decrease in the number of dopamine receptors in the striatum (parkinsonism “plus”).
Forms
There are two main types of parkinsonism:
- Primary
. This is Parkinson's disease, an independent disease. May be associated with other diseases, but not caused by them. - Secondary
. Occurs against the background of injury, poisoning, or other disease. It is a consequence, not a cause. Accordingly, treatment requires a different approach.
There are several forms of secondary disease, which are classified based on the preconditions of the disease.
- Infectious parkinsonism - develops after an infection. Most often, encephalitis, influenza, and syphilis act as provoking diseases.
- Vascular - develops in the post-stroke period. May be caused by chronic cerebrovascular accident.
- Toxic – a consequence of ingestion or exposure to various toxins. One of the forms - medicinal - develops against the background of taking pharmaceutical drugs.
- Traumatic – consequences of a head injury. It can occur with regular head injuries, for example, in athletes - wrestlers, boxers.
- Tumor - caused by brain tumors.
- Idiopathic is the name given to diseases whose cause has not been reliably determined. If there is a complete symptom complex of Parkinson's syndrome, then this diagnosis is made, despite the absence of an obvious cause.
General principles for starting treatment
Since at the moment the ability to slow down the process of degeneration due to the neuroprotective effect (the ability to protect intact cells from damage) or the neuroreparative effect (the ability to restore the activity of partially damaged cells) has not been convincingly proven in any of the drugs used, treatment is still based on symptomatic action. However, the potential for a neuroprotective effect, supported by experimental or clinical data, should be taken into account when prescribing treatment.
Currently, a widely accepted concept emphasizes the importance of early administration of dopaminergic therapy - immediately after diagnosis - in order to more quickly correct neurochemical imbalances in the brain and support compensation processes.
If previously the need for maintaining monotherapy as long as possible was emphasized, at present the advantages of this approach do not seem obvious - compared with an early transition to a combination of drugs with different mechanisms of action. The need for monotherapy or combination therapy should be decided individually. In any case, when choosing drugs and their dosage, one should strive not for complete elimination of symptoms, but for a significant improvement in function, allowing one to maintain everyday and professional activity. At the same time, you should avoid making several changes to the treatment regimen at once (for example, increasing the dose of several drugs at once or adding several drugs at once), this allows you to separately evaluate the effectiveness and safety of each of the prescribed drugs.
Signs and symptoms
How to spot Parkinson's disease?
The manifestation of the disease is characterized by a persistent loss of control over one’s own physical activity:
- Tremor at rest.
- Lack of mobility and muscle stiffness (rigidity).
- Reduced speed and insufficient range of motion.
- Insufficient ability to maintain balance (instability).
Rest tremor is involuntary small movements that occur in the absence of motor activity. The most common are sudden shaking of the hands and tilted movements of the head.
Signs not related to movement and coordination:
- Excessive fatigue.
- Manifestations of depression.
- Smell disorders.
- Excessive salivation.
- Increased sweating.
- Metabolic disorders.
- Negative changes in the functioning of the stomach and intestines.
- Psychoses and other psychological disorders.
- Degeneration of cognitive functions.
The most noticeable deviations in cognitive functions in parkinsonism are:
- Memory problems.
- Slowness of thought processes.
- Problems with orientation in space.
Principles for choosing an antiparkinsonian drug
The choice of drug at the initial stage of treatment is carried out taking into account age, severity of the motor defect, labor status, state of neuropsychological functions, the presence of concomitant somatic diseases, and the individual sensitivity of the patient. In addition to achieving optimal symptom control, the choice of drug is determined by the need to delay the development of motor fluctuations and dyskinesias (Table 2).
Table 2. Choice of drug for initial treatment of Parkinson's disease.
| Drugs | Can be used as first choice | Degree of symptomatic improvement | Neuroprotective potential | Risk of side effects | |
| Fluctuations and dyskinesias | Other side effects | ||||
| Levodopa | + | +++ | +? | ↑ | ↑ |
| Dopamine receptor agonists | + | ++ | +? | ↓ | ↑ |
| MAO B inhibitor | + | + | +? | ↓ | ↑ |
| Amantadine | + | + | +? | ↓ | ↑ |
| Anticholinergics | — | + | — | ? | ↑ |
In persons under 50 years of age with mild or moderate motor impairment in the absence of severe cognitive impairment, one of the following drugs is prescribed: dopamine receptor agonist, monoamine oxidase type B inhibitor, amantadine. For a milder motor defect, an MAO B inhibitor may be prescribed; for a more severe defect, it is preferable to start with treatment with one of the dopamine receptor agonists. Non-ergoline agonists (eg, pramipexole, ropinirole, rotigotine or pronoran) are preferred over ergoline agonists (bromocriptine, cabergoline) due to their more favorable side effect profile. If one of the dopamine receptor agonists is insufficiently effective or poorly tolerated, another dopamine receptor agonist or a drug from a different pharmacological group can be tried. A combination of a dopamine receptor agonist, MAO type B inhibitor and amantadine is rational, which should be switched to gradually, adding a drug of a new group if the previously prescribed drug did not provide the expected effect.
Anticholinergic drugs (for example, biperiden) are indicated in the presence of severe resting tremor or painful dystonia, provided that neuropsychological functions are preserved. It is advisable to add them to the combination of a dopamine receptor agonist with an MAO B inhibitor and/or amantadine, if in a relatively young patient it did not suppress tremor to the extent necessary to maintain his ability to work.
If these drugs in maximum tolerated doses and their combination do not provide an adequate state of motor functions and social adaptation of patients, levodopa is prescribed in the minimum effective dose [4].
In persons aged 50–70 years with a moderate motor defect and relative preservation of cognitive functions, treatment begins with an MAO type B inhibitor (for mild symptoms of parkinsonism) or one of the dopamine receptor agonists. In the future, it is advisable to gradually switch to a combination of a dopamine receptor agonist, an MAO type B inhibitor and amantadine (provided that it is well tolerated). Anticholinergics should generally not be prescribed to patients over 60 years of age due to the risk of cognitive decline and other side effects. If the combination of the above drugs is insufficiently effective, levodopa is added in the minimum effective dose (200–400 mg per day).
In persons aged 50–70 years with a pronounced motor defect that limits the ability to work and/or the ability to self-care, as well as in the presence of severe cognitive impairment and the need to obtain a quick effect, treatment begins with drugs containing levodopa. If small to moderate doses of levodopa (300–500 mg levodopa per day) do not provide the necessary improvement, a dopamine receptor agonist, amantadine, and an MAO B inhibitor may be added sequentially.
In elderly people (over 70 years of age), especially in the presence of severe cognitive decline and somatic burden, treatment should begin with levodopa. The age limits indicated are relative, and the general principle is rather that the younger the patient, the later levodopa should be administered. In addition, it is not so much the chronological, but the biological age of the patients that plays a decisive role.
What is Parkinson's disease
Parkinson's disease is a neurodegenerative brain disease that develops slowly in most cases. Over the course of many years, the symptoms of this pathology may progress unnoticed. Due to the death of a significant number of neuronal cells in areas of the basal ganglia and degradation of nerve fibers, this disease occurs. Symptoms of Parkinson's disease begin to actively manifest themselves when the functions of 80% of neural connections are impaired. In such cases, Parkinson's disease is incurable and develops over time, despite all the therapy taken.
Neurodegenerative diseases are a group of diseases that develop slowly and are characterized by hereditary or acquired lesions of the central nervous system.
Another specific feature of Parkinson's disease is a sharply reduced amount of dopamine. Its quantity is no longer sufficient for the necessary inhibition of nerve impulses flowing from the cerebral cortex. Thanks to this, nerve impulses go straight to muscle tissue, stimulating its tension. This is typical for the most striking manifestations of Parkinson's disease: muscle contractions (trembling and tremors), stiffness of muscle activity due to excessively high tone, manifestations of unintentional body movements.
Use of extended-release pramipexole in early PD
The development of new dosage forms of antiparkinsonian drugs that provide them with a prolonged release and allow a single dose during the day not only makes treatment more convenient, but also, by improving patient adherence to treatment, increases the long-term effectiveness of therapy. In addition, with a slow release of the drug throughout the day, a more stable concentration in the blood is achieved, which can ensure better tolerability and effective control of disease symptoms throughout the day (both during the day and at night).
A new dosage form of pramipexole with prolonged (controlled) release, which involves a single dose during the day, has been used in European countries and the USA since 2009, and in our country - since 2012. It is a matrix tablet in which the active substance is evenly distributed in a polymer matrix. In the gastrointestinal tract, the matrix absorbs liquid and turns into a gel, which uniformly releases pramipexole over 24 hours. Since pramipexole is highly soluble in a liquid medium, regardless of its pH, the active substance is released from the matrix and absorbed throughout the intestine. The rate of gastric emptying and intestinal motility do not significantly affect the effect of the drug. Absorption parameters also do not depend on whether the drug is taken on an empty stomach or after a meal [2].
When developing a new dosage form, the possibility of a simple, immediate transition from the traditional form of the drug to the new one was taken into account. The condition for this is that equal daily doses of immediate release (taken 3 times a day) and extended release (taken once a day) have the same antiparkinsonian effect. The difference between the new and traditional dosage forms of pramipexole lies only in the rate of release of the active substance. The half-life of pramipexole is the same when using both forms, but controlled release ensures longer maintenance of therapeutic drug concentrations in the blood [5].
The equivalence of equal daily doses of immediate- and extended-release pramipexole has been demonstrated in a number of clinical trials.
It is worth emphasizing the particular convenience of the new dosage form of pramipexole, which can be taken once a day, for patients with early stage PD who continue to work. To avoid side effects, the drug is prescribed by slow titration - according to the same scheme as the immediate-release drug. For this purpose, extended-release pramipexole tablets are available in several strengths: 0.375, 0.75, 1.5, 3 and 4.5 mg. Treatment begins with a dose of 0.375 mg once a day, then, subject to good tolerance, every 7 days they move to the next dose level until the optimal effect is achieved, up to a maximum of 4.5 mg/day (Table 3). After reaching a dose of 1.5 mg/day, it is sometimes advisable to titrate more slowly, since the development of the full therapeutic effect may require several weeks. The recommended dose for maintenance therapy (both in early and in advanced or late stages of the disease) can range from 0.375 to 4.5 mg/day. The most commonly used dose is 3 mg/day.
Table 3. Titration schedule for extended-release pramipexole.
| A week | Dose |
| 1st | 0.375 mg 1 time per day |
| 2nd | 0.75 mg 1 time per day |
| 3rd | 1.5 mg 1 time per day |
| 4th | 2.25 mg 1 time per day |
| 5th | 3 mg 1 time per day |
| 6th | 3.75 mg 1 time per day |
| 7th | 4.5 mg 1 time per day |
How is Parkinson's disease treated?
- In the early stages of Parkinson's disease, it is treated with medications, introducing substances that are lacking into the body. The main target of drug (chemical) therapy is the “substantia nigra”. When using this method of treatment, almost all patients experience a noticeable reduction in symptoms and they have a lost opportunity to live a normal life.
- But there are cases when patients do not improve, even after taking medications for a long time and increasing their dosages. In such cases, brain surgery is used to implant a stimulator into the brain.
This operation takes the form of high-frequency stimulation of the basal ganglia with electric current through an electrode connected to an electrical stimulator:
- When using local anesthesia, two electrodes are sequentially introduced into the patient’s brain (along a path determined in advance by a computer), producing deep stimulation of parts of the brain.
- Under anesthesia, an electrical stimulator connected to electrodes is implanted into the chest area under the skin.
Medicines that have not been proven to be effective in PD
In clinical practice, medications are widely used whose effectiveness in PD has not been proven and which, therefore, cannot be recommended for use in this disease. First of all, these include the so-called nootropic, neurometabolic and vasoactive drugs. It is possible that some of these drugs have some therapeutic effect, but before recommending a specific drug, an adequate assessment of its effectiveness should be carried out. Specialists involved in the treatment of PD are well aware that a certain proportion of patients respond well to placebo, and this effect is not durable. Accordingly, the costs of such treatment turn out to be meaningless.
Literature
- Golubev V.L., Levin Ya.I., Vein A.M. Parkinson's disease and parkinsonism syndrome. M.: MEDpress-inform, 1999.
- Levin O.S., Fedorova N.V., Smolentseva I.G. Dopamine receptor agonists in the treatment of Parkinson's disease. Rus. honey. magazine 2000; 15–16: 643–646.
- Levin O.S., Fedorova N.V. Parkinson's disease. M.: MEDpress-inform, 2011.
- Patient management protocol. Parkinson's disease. Problem standardization in healthcare. 2005; 3: 74–166.
- Chwieduk CM, Curran MP Pramipexole extended release in Parkinson's disease. CNS Drugs 2010; 24: 327–336.
- Grosset KA, Bone, Grosset DG Suboptimal medication adherence in Parkinson's disease. Mov. Discord. 2005; 20: 1502–1507.
- Hauser R., Salin L., Koester J. Double-blind evaluation of pramipexole extended-release (ER) in early Parkinson's disease. Neurology 2009; 72(11 Suppl. 3):A412–413.
- Kvernmo T., Härtter S., Bürger E. A review of the receptor-binding and pharmacokinetic properties of dopamine agonists. Clin. Therap. 2006; 28:1065–1078.
- Mizuno Y., Yamamoto M., Kuno S. et al. Efficacy of Pramipexole Extended Release (ER) and switching from Pramipexole Immediate Release (IR) to ER in Japanese advanced Parkinson's disease (PD) patients. In: XVIII WFN World Congress on Parkinson's Disease and Related Disorders: Miami Beach, 2009: Poster 2.192.
- Möller JC, Oertel WH Pramipexole in the treatment of Parkinson's disease: new developments. Expert Rev. Neurother. 2005; 5:581–586.
- Poewe W., Rascol O., Barone P. et al. Pramipexole extended-release in early Parkinson's disease. Neurology 2011; 77:759–766.
Drugs used for treatment:
"Amantadine" or "Midantan". These are the drugs used to start the treatment of Parkinson's disease. This medication stimulates the release of dopamine, helps reduce its reuptake by blocking glutamate receptors, protects the “substantia nigra,” and also has other positive properties. Amantadine significantly reduces the manifestations of hypokinesia and rigidity (stiffness and limited movement), however, it almost does not reduce tremor and trembling. These medications are well tolerated and have almost no side effects.
Rotigotine or Newpro. It is an agonist of the dopamine receptor group. Available in the form of a patch that is applied to the skin. This patch is a transdermal therapeutic system with dimensions of 10-40 cm², used once a day. Such medications are sold exclusively by prescription and are used for independent (without combination with other drugs) treatment of Parkinson's disease in the early stages.
The use of this form of drugs has significant advantages: the dosage is smaller, but more effective, and the side effects are less pronounced.
"Leftfall." It has long been considered one of the best medical drugs for the treatment of Parkinsonism. This compound is a chemical precursor to dopamine. But it is worth remembering that this substance has a significant number of side effects, such as psychological disorders. It is prescribed, as a rule, in combination with “carbidopa” or “benserazide”. They lead to better absorption of “levodopa” and reach areas of the brain and help reduce side effects.
"Modopar". It is a combination drug containing both “leovdopa” and “benserazide”. Produced in various forms. "Madopar GSS" is produced in special capsules, the shell density of which is significantly less than that of gastric juice. This ensures that the substance enters the body slowly and evenly, because such a capsule dissolves in the stomach from 5 to 12 hours. “Dispersible Madopar” is also available, which has a liquid form, thanks to which it acts much faster. This option is more suitable for patients with limited swallowing function.
"Miralex". This drug is produced as tablets, which are used both as a stand-alone drug in the early stages of the disease, and as a combination therapy with levodopa in the later development of Parkinson's disease. Miralex has fewer side effects than non-selective agonists, but at the same time more than amantadine. Such side effects can manifest themselves as nausea, pressure surges, excessive drowsiness, changes in the balance of enzymes secreted by the liver, and swelling of the lower extremities. Hallucinations are also possible in patients diagnosed with dementia.
"MAO inhibitors." They significantly slow down the oxidation of dopamine, located in the “striatum” of the brain, due to this its concentration in synapses increases. The most common use in the treatment of the disease is Selegiline. In the first stages of Parkinson's disease, Selegiline is used as an independent therapy (without the use of other combination drugs). Many patients report significant improvements when using these products. Undesirable effects from the use of Selegiline are rare and mild.
Treatment with Selegiline can delay the prescription of lepodopa for a period of 9 to 12 months. In the last stages of the disease, Selegiline is used as an addition to Levodopa, which allows the latter to increase its effectiveness by a third.
"Mydocalm." This drug greatly reduces the abnormally large tone of muscle tissue. This property is used in Parkinson's disease as an auxiliary. "Mydocalm" is available in tablets, for oral use and ampoules, for intravenous and intramuscular use.
"B vitamins." This group of vitamins is often used to treat diseases of the central nervous system. In order for L-Dopa to be safely converted into dopamine, nicotinic acid and vitamin B6 are needed. Also, “Thiamin” (vitamin “B1”) is used to increase the content of dopamine in parts of the brain.
Treatment
Disadvantages of official therapeutic methods for treating Parkinson's disease
Unfortunately, of all the treatment options for this disease, there is practically no effective treatment method, and drug therapy has only a symptomatic effect, that is, it affects more the consequences of the disease that has arisen, which cannot even slow down the processes, but has a number of side effects that sometimes aggravate the process . In addition, with long-term (years) use of the drug, addiction occurs to it; in this case, it is necessary to increase the dose of the drug or change the drug itself, otherwise the symptoms of the disease will intensify. It is important to adequately and accurately select the drug itself and correctly determine the dosage, but, unfortunately, therapy is often carried out blindly, and to achieve a therapeutic result, several medications are taken at the same time, which depletes the body’s capabilities and reduces defense mechanisms. As a result, the form becomes more severe and the disease progresses.
One of the main causes of parkinsonism is considered to be a decrease (with age) in the number of pathways in the central nervous system, which leads to an imbalance of neuro-reflex excitability, which must be suppressed. Existing medications can provide such inhibition, but this often worsens overall health.
Apitherapy
Treatment goals for Parkinson's disease:
- impact on predisposing factors;
- replenishment of dopamine deficiency from the outside;
- stimulation of one’s own production of neurotransmitters, in particular dopamine;
- selective impact on reflex-motor mechanisms;
- symptomatic treatment;
- complete comprehensive rehabilitation.
To accomplish these tasks, no existing medication or even a group of medications is enough. However, having studied this disease for more than 20 years, which belongs to the group of diseases that are difficult to treat with existing medical methods, we came up with a substance that can cover all of the above - this is BEE VENOM, more precisely the components of the poison - APITOXINS, and on their basis we developed the “APITOX” program.
Treatment and rehabilitation apitoxin therapy for Parkinson's disease, within the framework of the "APITOX" program.
So, to obtain results in the treatment of this disease, you need a comprehensive and thoughtful approach with competent definition of goals:
- Creation of an adequate level of dopaminergic activity;
- Improving nutrition of brain tissue;
- Correction of neurological symptoms and motor activity;
- Stabilization of the psycho-emotional background.
To achieve these goals, correctly structured tasks are important:
- Reducing or stopping the progression of the disease;
- Achieving a positive result by increasing the production of DOPA and catecholamines (dopamine, adrenaline, norepinephrine);
- Asymptomatic treatment;
- Motor rehabilitation;
- Psycho-emotional rehabilitation;
- Improving quality of life.
An improvement in the condition and a decrease in the symptoms of the disease is recorded in almost all patients, and those with muscle rigidity turned out to be better amenable to apitoxin therapy.
Positive dynamics were achieved not only in the clinical picture at a given time, but also in the future, due to the fact that bee venom is able to affect all structural components of Parkinson’s disease (causal factors and mechanisms of formation).
Replenishment of the missing dopamine is achieved due to the direct content of this neurotransmitter in bee venom. Also, the components of bee venom promote the release of biogenic amines: dopamine, serotonin, etc., which normalizes the concentration of dopamine.
With atherosclerotic changes in blood vessels, in combination with arterial hypertension and dyscirculatory encephalopathy, the components of bee venom, due primarily to its component cardiopep, have a vasodilating effect and promote the accumulation of prostaglandins in the connective tissue. In turn, melittin, which is part of the poison, has a strong blood thinning property and also dissolves blood clots. All this contributes to a significant improvement in cerebral circulation and blood pressure. Along with the above effects, “working” through BAP, bee venom normalizes the passage of nerve impulses along the nerve processes, and also restores trophic processes due to the content of 18 essential amino acids.
Obtaining a therapeutic effect in PD is possible regardless of the symptoms of the disease and the duration of the disease. This occurs due to the effect of apitoxins on all structural components and the extensiveness of the “APITOX” treatment program, in which a large group of specialists with different specializations takes part, but all working under the same control.
Signs
Clinically significant signs of the disease:
- decrease in motor activity - hypokinesia;
- decreased muscle elasticity - rigidity;
- tremor (shaking);
- imbalance - postural disorders.
1. Hypokinesia (bradykinesia) combines a decrease and slowdown in a person’s motor activity. Patients feel the symptoms of this disease as a feeling of weakness and increased fatigue, and from the outside, the most noticeable thing is a significant decrease in facial expressions, a decrease in gestures in conversation. The greatest difficulty for a patient with this disease is the initial period of motor activity, as well as coordinated complex movements. The patient walks in small steps, with the legs placed straight and close to each other. This gait is called “puppet gait”. A special posture for Parkinson's disease is characteristic in the form of hunching, tilting the torso and head forward, arms brought towards the body, bent at the elbow joints at an angle of 90, legs bent. As this disease progresses, patients pay attention to the intensification of this condition in the evening, which is manifested by severe stiffness of movements at night, as a result of which it is impossible to change the position of the body during sleep. In the morning, it is difficult for the patient to get out of bed without outside intervention.
The decrease in movements is more pronounced in the body, lower and upper extremities, and facial muscles. Their speech is inexpressive and monotonous.
2. Rigidity is a condition involving muscle tension, especially in the limbs. When examining a person with Parkinson's disease, the “cogwheel” symptom, which is specific to this condition, is revealed. It is manifested by muscle resistance when bending the arms and legs. Increased tone of the muscles of the back and chest is another symptomatic condition of the patient, the so-called “petitioner” pose - a significant stoop in the thoracic spine with a forward tilt of the torso. Muscle tension can be accompanied by intense pain, and sometimes this is what prompts the patient to seek medical help for the first time. Sometimes these painful sensations are misdiagnosed and can be disguised as symptoms of rheumatism, which is why the diagnosis is sometimes made incorrectly.
3. Shaking or tremor is an optional sign of Parkinson's disease. In general, small-scale trembling in this condition is special and differs from trembling in other diseases. It occurs at rest and appears only in one hand; when the disease intensifies, it appears on the neighboring arm and legs. The mechanism of its formation is the rhythmic contraction of muscle fibers acting opposite to each other with a frequency of 5 vibrations per minute.
The specific opposite movements of the first and other fingers of the hands are reminiscent of the movements of the hands when counting coins. This symptom is called “pill rolling” or “counting coins.” Rarely, a small-scale tremor of the head forward and backward - “yes-yes” or to the sides “no-no”, trembling of the eyelids, tongue, and lower jaw can be observed. The peculiarity of this variant of trembling is that it increases with anxiety and disappears during sleep and spontaneous movements. These are the main distinguishing features of tremor in Parkinson's disease - its reduction or disappearance with purposeful movement.
It is very similar to parkinsonian tremor in the clinic - the tremor is essential, but its distinctive feature is its constancy even with purposeful movements.
4. Postural instability is a kind of clumsiness in walking due to difficulties in overcoming (difficulty starting and finishing walking). Today it is a mandatory symptom of this disease, like tremor, rigidity and hypokinesia. These reflexes are responsible for the process of standing, starting and ending walking. These are unconscious reflex movements. In Parkinson's disease, there is a discoordination of these reflex acts, as a result of which the human body in the process of movement is ahead of the legs and it is as if the body is being pushed forward of the legs.
It is precisely because of these violations of the “stability” reflexes that the frequent falls of patients with Parkinson’s disease are explained.
Symptoms
Symptoms of Parkinson's disease include:
- Disruption of the autonomic nervous system - increased salivation, increased oiliness of the skin of the face and hair, dandruff of the scalp, increased sweating or, conversely, dry skin, difficulty urinating, constipation, decreased libido.
- Changes in mental state (appear spontaneously). Initially, changes appear regarding certain areas of life, manifested by an increase in certain character traits: the patient develops pettiness, scrupulousness, excessive meticulousness, difficulty in switching from one topic of conversation to another, becomes grumpy, and conflictual. Unreasonable mood swings appear, interest in life decreases, clinginess (akairia), slowness and rigidity of thinking appear. The patient withdraws into himself, strives to limit himself in communication with other people, and sometimes becomes embittered. Parkinson's disease worsens the quality of life, there is an emotional and volitional decline, and may be responsible for the occurrence of depression. Memory is significantly reduced. Mental abilities increase over time and dementia develops. Another unfavorable factor is that medications used in the treatment of Parkinson's disease provoke the occurrence of mental disorders such as hallucinations and psychosis.
- Changes in the sleep process - difficulty falling asleep, a feeling of “lack of sleep”, shallow sleep with frequent awakenings. Part of the reason for the occurrence of these disorders is the symptoms of a painful condition: stiffness of movement does not allow changing the position of the body in bed.
- Symptoms of sensory changes: hyperesthesia, local pain, numbness of certain areas, crawling sensation, restless legs syndrome, etc.
A classification that evaluates the patient's motor activity and ability to perform independent maintenance was proposed in 1967 by Hoehn and Yahr:
- Stage 0 – no movement deviations.
- Stage 1 – impaired motor function on one side only.
- Stage 2 – impaired motor function on both sides, but without deviations in postural reflexes.
- Stage 3 – moderate degree of deviation of postural reflexes, but can take care of itself independently.
- Stage 4 – significant difficulties in movement, but movement without assistance.
- Stage 5 – significant motor impairment requiring additional care for the patient.
In addition, Parkinson's disease is divided according to the time of its onset:
- Juvenile form - debut at the age of 13-14 years.
- The early form debuts between the ages of 20 and 40.
- Late-onset form - after 50 years.
There are the following forms of Parkinson's disease:
- The form is trembling-rigid. Its main characteristic symptom is tremor. It is detected in 37% of cases.
- The shape is akinetic-rigid. A distinctive sign of this condition is muscle stiffness and lack of mobility. Tremor is either absent or appears slightly in exciting situations. Occurs in 33% of cases of the disease.
- The form is rigid and trembling. A type of pathological condition is slowness and excessive muscle tension. Occurs in 21% of cases.
- The form is trembling. From the very beginning, the disease manifests itself as tremor, and this symptom remains the main one. Other manifestations of Parkinson's disease: muscle tone is not increased, slowness and amicability are weakly expressed. This form makes up only 7% of all forms.
- The form is akinetic. The main symptom of the condition is a decrease or virtual absence of spontaneous movements. This type occurs in only 2% of cases.
The akinetic form of the disease is manifested by a high degree of decreased movements with a significant decrease in general motor activity and gestural movements. Facial expressions become poorer, they become slower and less expressive, and in severe cases the face “freezes” and becomes hypomimic. Patients with Parkinson's disease experience decreased blinking - Marie's syndrome. Slowness can be seen even in speech; it becomes less emotionally charged and muffled. During walking, a phenomenon called acheirokinesis is observed - the absence of friendly hand movements. The gait in such patients is small steps with the torso tilted forward and, as if at the beginning of walking, running a little - the phenomenon of propulsion.
In the akinetic-rigid form of the disease, significant muscle rigidity is revealed along with decreased movements. Along with increased muscle tension (hypertension), a specific symptom is determined - “cogwheel” - intermittent resistance to passive flexion and extension of the arms and legs. Muscle rigidity is detected either in all muscle groups of the limbs and body, or is local in nature, affecting individual limbs. Significant muscle rigidity in a person with Parkinson's disease manifests itself in the “supplicant pose”: the body is tilted forward, the head is lowered down, the chin is brought to the chest, the arms are pressed to the body, the elbows are bent, the hands are clenched.
The rigid-tremorous form of the disease combines rigidity and tremulous hyperkinesis in the clinical picture. The tremor is characterized by small rhythmicity, especially at rest (rest tremor), more in the hands, and is not detected during purposeful movements. Parkinsonian tremors are characterized by monotonous movements in the form of adduction of the thumb, as if rolling pills. In other cases, the tremor spreads to the muscle groups of the neck and head. But still, the basic symptom of the tremulous form of parkinsonism is tremulous hyperkinesis, and akinesia and muscle rigidity are less pronounced.