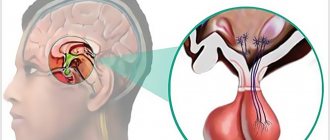
The hypothalamic-pituitary system determines the functional state of the entire endocrine system. The anatomical and functional relationship of the hypothalamus and pituitary gland also ensures the unity of the nervous and endocrine systems.
The hypothalamus (hypothalamus) occupies part of the diencephalon. As an autonomic center, the hypothalamus coordinates the function of various systems and organs, regulates the function of the endocrine glands (pituitary, ovaries, thyroid and adrenal glands), metabolism (protein, fat, carbohydrate, mineral and water), temperature balance and the activity of all body systems ( vegetative-vascular, digestive, excretory, respiratory, etc.).
This multifaceted function of the hypothalamus is provided by neurohormones entering it through the portal vascular system after being released from the endings of the hypothalamic nerve fibers.
The thyroid gland and its hormones
The thyroid gland and its hormones, together with the nervous and immune systems, take part in the coordination and regulation of the work of all human organs (heart, brain, kidneys, etc.).
In a coordinated “orchestra” of signals, nerve impulses and biological substances, thyroid hormones play the role of the “main violin”. The reason for the special importance of thyroid hormones for the body is that they are needed by all tissues and every cell. Simply put, it is impossible to exist without them.
The role of the thyroid gland is so significant that its study is separated into a separate discipline - thyroidology, and after the accident at the Chernobyl nuclear power plant and Fukushima, it is under close attention.
The problem of imbalance of thyroid hormones has been known for many centuries. Ancient Roman doctors were the first to draw attention to the increase in its size during adolescence and pregnancy. Before our era, China already knew how to prevent the appearance of goiter - an enlarged gland - by eating seaweed. In the Renaissance, a rounded and swollen neck was a sign of female attractiveness, which Rembrandt, Durer, and Van Dyck emphasized in their paintings. A nervous and excitable disposition, as a result of an excess of thyroid hormones, was in fashion in the 17th century in Spain. Calm and graceful slowness was valued by the aristocrats of Switzerland, but they did not suspect that the reason for this was a deficiency of iodine, necessary for the thyroid gland.
Diseases of the hypothalamic-pituitary system
Symptoms, diagnosis, therapy and prevention of diseases of the hypothalamic-pituitary system at the Diadent clinic.
Wide range of diagnostic methods, carefully selected treatment. Make an appointment at the clinic.
free consultation
Discount 500 ₽!
When making an appointment for treatment with specialists from the Diadent Group of Clinics, use the “last minute” windows of the current day (does not apply to consultations). Check with the administrators for the list of “burning” windows.
cost of endocrinology services
Consultation
- 1,500 rub. Primary consultation with an endocrinologist
- 1,500 rub. Repeated consultation with an endocrinologist
Tests and diagnostics
- 1,500 rub. Performing a glucose tolerance test
- 1,600 rub. Ultrasound of the thyroid gland and parathyroid glands
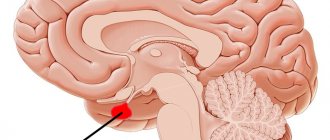
The hypothalamic-pituitary system is a complex of structures consisting of two sections and their connections. The components of this system are the pituitary gland, hypothalamus and stalk.
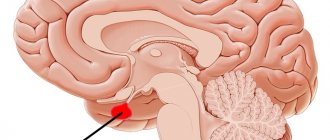
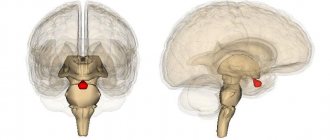
The pituitary gland is a lower cerebral appendage located in the area of the sella turcica, which is the central organ of the endocrine system of the human body.
Hypothalamus , located above the pituitary gland and below the thalamus, is part of the diencephalon responsible for autonomic function. The stalk, or instep, is the area connecting the hypothalamus to the pituitary gland.
The hypothalamic-pituitary system connects the nervous and endocrine systems of the body with each other, regulating the synthesis of hormones, without which the correct functioning of many organs is impossible. Each of the departments included in the system complex produces a special type of hormones that have a direct effect on the activity of organs and systems.
What are the hormones of the pituitary gland and hypothalamus responsible for?
The hormones of the pituitary gland and hypothalamus closely interact with each other. The functions of our body such as:
- feeling of hunger and satiety;
- feeling of thirst;
- natural thermoregulation;
- maintaining the circadian rhythm of sleep and wakefulness;
- feeling of need for procreation;
- manifestation of aggression;
- the ability to experience different emotions.
Hormones of the hypothalamic-pituitary system
The main function of the pituitary gland is the production of hormones involved in the regulation of the activity of peripheral endocrine glands. These hormones are responsible for reproductive functions, tissue growth, metabolic processes in the body, and ensure the normal functioning of the thyroid gland.
The hypothalamus produces releasing hormones, liberins and statins , which support the functioning of the adrenal glands, thyroid and pancreas and reproductive system, as well as those involved in the processes of regulating body temperature and supporting the normal functioning of internal organs.
Any disturbances in the functioning of the hypothalamic-pituitary system can result in extremely negative consequences, even death. Unfortunately, quite often the pathologies of this system develop latently, manifesting themselves only in the form of concomitant diseases, which, at first glance, are not related to the functioning of the endocrine and nervous systems.
Find out the cost of treatment at a free consultation by phone +7 (812) 40-000-60
your benefit of treatment with us
- Any payment methods We offer you many ways to pay for the clinic’s services for individuals and legal entities: cash and bank transfer, card or using a certificate.
- No queues
Make an appointment and it will take place at exactly the appointed time. - Complete safety of procedures
Diadent has developed a perfect safety system that reduces the risk of disease (protection against HIV, hepatitis and other diseases) to zero.
Diagnosis and treatment of diseases of the hypothalamic-pituitary system
A comprehensive examination may include:
- anamnesis analysis;
- visual inspection;
- MRI;
- CT;
- radiography of the sella turcica;
- spinal puncture;
- blood tests for hormones - pituitary gland and hypothalamus, adrenal glands, thyroid gland and sex hormones.
Only after receiving accurate data on the state of the entire system as a whole and each of its components separately, an endocrinologist can accurately diagnose and prescribe appropriate treatment that will normalize the functioning of the pituitary gland, hypothalamus and the entire body.
To assess the functioning of the system, a blood test is performed for a complex of hormones involved in the functional connection of its elements. If the development of hormonally inactive adenomas is suspected, a blood serum test for the concentration of chromogranins is used.
Treatment of diseases of the hypothalamic-pituitary system is strictly individual, directly depends on the diagnosis and associated factors, such as the degree and severity of the disease, the presence of allergic reactions, intolerance to certain medications, gender and age of the patient, etc.
It is important to remember that the earlier treatment is started, the lower the likelihood of developing serious complications, which often pose a serious threat to the patient’s life.
Find out the cost of treatment at a free consultation by phone +7 (812) 40-000-60
Where is Diadent Clinic located?
Multidisciplinary clinic
St. Petersburg, Bukharestskaya st. 110, building 1
Phone: + 7 (812) 40-000-60
To assess the functioning of the system, a blood test is performed for a complex of hormones involved in the functional connection of its elements. If the development of hormonally inactive adenomas is suspected, a blood serum test for the concentration of chromogranins is used.
Treatment of diseases of the hypothalamic-pituitary system is strictly individual, directly depends on the diagnosis and associated factors, such as the degree and severity of the disease, the presence of allergic reactions, intolerance to certain medications, gender and age of the patient, etc. It is important to remember that the earlier treatment is started, the lower the likelihood of developing serious complications, which often pose a serious threat to the patient’s life.
Structure of the thyroid gland
The thyroid gland is located on the front of the neck, just below the Adam's apple. The ancient Roman physician Galen first described the gland as a separate organ, and it received its name much later in the 17th century. The name of the gland comes from the Greek words “thyreos” - shield and “idos” - view, i.e. an organ that looks like a shield. The international name for this internal secretion organ is the thyroid gland. The shape of the thyroid gland resembles a butterfly or a horseshoe; it has three main parts - two lateral lobes and an isthmus. Every third person has another non-permanent lobule - pyramidal.
The size of the gland can vary significantly even in the same person, depending on the activity of its functioning. Gender, age, climate, medication use and, of course, diet largely influence the size and amount of gland hormones. Due to the tight connection with the larynx, its position can change, it rises and falls when swallowing, moves to the side when turning the head in different directions, which is visible to the naked eye
The structure of the thyroid gland is quite complex. Under a microscope, it is noticeable that it consists of many vesicles - follicles. Along the edges of the follicles there are cells - thyrocytes, and inside the follicle there is a thick watery liquid - colloid. Thyrocytes synthesize hormones, and they accumulate in a colloid for immediate release into the blood when needed.
In the walls of the follicles between the cells, as well as between the follicles themselves, there are larger, lighter parafollicular cells (C-cells), which produce the hormone calcitonin, which is involved in the regulation of calcium and phosphorus metabolism. It inhibits the removal of calcium from bones and reduces calcium levels in the blood.
Thyroid hormones
The main two hormones produced by the thyroid gland are triiodothyronine (contains three iodine molecules) and tetraiodothyronine or thyroxine (contains four iodine molecules). Thyroid hormones are abbreviated as T3 and T4. In the cells and tissues of the body, T4 gradually turns into T3, which is the main biologically active hormone that directly affects metabolism.
The formation of thyroid hormones is associated with a specific protein, thyroglobulin. Thyroglobulin serves as a reserve form of thyroid hormones and is located inside the colloid.
In the preparation of thyroid hormones, two essential components are required - iodine and the essential amino acid tyrosine. To form one T4 molecule, four iodine molecules are needed, while T3 requires only three. Without iodine, hormone synthesis stops completely. This is why it is so important to prevent iodine deficiency in food. Tyrosine enters the body with food; it is a precursor in the formation of not only thyroid hormones, but also adrenaline, melanin, and dopamine.
There are four stages in the process of synthesis of thyroid hormones:
1. Absorption of iodine by the thyroid gland. Its concentration in the gland is 30-40 times higher than in the blood.
2. Activation of iodine, which makes it possible for it to bind to the amino acid tyrosine molecule.
3. Condensation with the formation of hormones - thyroxine and triiodothyronine and their accumulation in the composition of thyroglobulin in the form of a colloid.
4. Release of formed hormones into the blood under the influence of TSH.
Thyroid hormones are very small in size and must be bound to transport proteins before entering the blood so as not to be “washed out” of the body by the kidneys. The level of free hormones is 0.03% of the total; they provide all the effects of thyroid hormones. In tissues, thyroxine (T4) is converted into triiodothyronine (T3) and the biological effect of hormones is 90% due to T3.
The mechanism of regulation of ovarian function in normal conditions and in cases of hypothalamic-pituitary dysfunction.
A symptom of infertility is a manifestation of the depletion of the compensatory capabilities of certain parts of the reproductive regulation system. In 50 - 70% of cases, infertility is determined by the condition of the wife, in 20 - 25% of cases - by the condition of the husband. In 10 - 30% of cases, mixed forms occur, and in 2 - 5% of cases the cause of infertility is not clear (2). In the structure of female infertility, endocrine disorders occur in 35 - 40% of cases, dysfunction of the fallopian tubes - in 30 - 40% of cases, uterine factors - in 10%, cervical - in 7 - 10% of cases, vaginal - in 6%, extragenital - in 1%, mental - in 1% of cases. The same or similar data are given in most gynecological manuals (10).
The basis of a woman’s reproductive system is the hypothalamus-pituitary-ovary axis, the proper functioning of which ensures the maturation of a full-fledged egg, adequate preparation of the endometrium for pregnancy, tubal transport of gametes, fertilization, implantation and maintenance of early pregnancy.
The highest organ of regulation of the hypothalamic-pituitary-ovarian axis is the central nervous system, which, through a whole complex of direct and feedback connections, ensures the stability of the reproductive system when the internal and external environment changes (14, 21). To date, more than 36 peptides have been discovered that regulate GnRH secretion (28). Based on the fact that all the main neuroendocrine circles are directly or indirectly connected with the immune system and, in addition to the endocrine centers, connect areas of the brain with lymphoid tissue, some researchers are currently talking not about the neuroendocrine, but about the neuro-immune-endocrine system for regulating reproduction (30, 33).
The releasing factor for the two major gonadotropins, LH and FSH, is Gn-RH, a decapeptide synthesized by Schally and Guillemin in 1977. GnRH is synthesized in the arcuate nucleus of the mediobasal hypothalamus and enters the portal blood flow system of the pituitary gland in a pulsed manner. To ensure normal secretion of gonadotropins, it is sufficient to maintain a stable frequency of release of physiological amounts of Gn-RH. Changing the frequency of Gn-RH release changes not only the amount of LH and FSH secreted by the pituitary gland, but also their ratio, while even a tenfold increase in the concentration of Gn-RH leads only to a slight decrease in FSH release and does not change LH secretion in any way.
The frequency of Gn-RH release in humans is 1 release every 70 - 90 minutes and corresponds to a number of biorhythms (alternation of sleep phases, fluctuations in glomerular filtration rate and gastric secretion, frequency of hot flashes during menopause, etc., which confirms Kleitmann's hypothesis about the existence of general rhythm with a periodicity of about 90 minutes, which is related to the basal rest-activity cycle (20), which is explained by geophysical reasons (22, 37).The main factors regulating the frequency of GnRH release are opiates and alpha-blockers (6, 12, 13).The pulse rhythm generator - the arcuate nucleus - does not need any influence from other parts of the nervous system to maintain its normal operation (1).Under physiological conditions, the pulse generator receives information about the release of gonadotropins by the pituitary gland through a short feedback system, since special sphincters regulate pressure gradients in the portal blood flow system, and part of the blood from the pituitary gland does not flow into the cavernous sinus, but back into the hypothalamus, which ensures a very high local concentration of pituitary hormones in the hypothalamus (31). The synthesis and secretion of LH and FSH in the pituitary gland are carried out by the same cells (7). On the surface of gonadotropes there are receptors for Gn-RH, the density of which depends on the level of steroid hormones in the blood and on the concentration of Gn-RH. The connection of Gn-RH with the receptor causes a massive influx of calcium ions into the cell, which after a few minutes leads to the release of LH and FSH reserves into the bloodstream. In addition, GnRH stimulates the synthesis of LH and FSH and maintains the integrity of gonadotropes (40). Changes in pulse generator frequency alter the ratio of LH and FSH secreted by the pituitary gland (24). Thus, an increase in rhythm leads to a significant increase in the release of FSH and a decrease in the release of LH. Frequency modulation of information ensures speed and reliability of regulation of the reproductive system and its resistance to interference (4, 36). In the luteal phase, progesterone, through endogenous opiates, reduces the frequency of the pulse generator, and this action is determined not by the concentration of progesterone, but by the duration of its effect. Estradiol, acting on the hypothalamus and gonadotropes (increasing the density of Gn-RH receptors), increases the amplitude of the LH / FSH wave (16, 39).
Progesterone stimulates the formation of an inhibitor in the hypothalamus, which eliminates this effect of estradiol (29, 35). This eliminates the possibility of an LH peak during the luteal phase, which could disrupt the maturation of a cohort of follicles for the next menstrual cycle (11).
Gonadotropins are the main regulators of the synthesis and secretion of sex steroids. The site of production of sex steroids in the body can be the follicular complex (theca interna, theca externa, granulosa and oocyte), the corpus luteum and the ovarian stroma. The usefulness of cyclic changes that ensure the preparation of a woman’s body for pregnancy is determined by the quality of selection and maturation of the dominant follicle. The main patterns of folliculogenesis were established by the working group of Professor Hodgen at the turn of the 1970s and 1980s (11). They proposed the terms recruitment, cohort, selection, establishment of dominance. Recruitment is the name given to the process of transition of follicles from the primordial stage to the antral stage, since only from this time the maturation process becomes dependent on the action of gonadotropins. The recruitment process is determined by intraovarian factors and occurs constantly, but only those follicles that are recruited in the last 4 days of the luteal phase of the previous cycle will be able to form a cohort - a group of follicles from which the dominant one will emerge (39). The number of recruited follicles is most likely determined by the level of gonadotropins in the late luteal phase and the local concentration of progesterone in the ovary, which explains the alternation of ovulation in the right and left ovaries. The growth of the cohort of follicles in the early follicular phase is explained by favorable conditions for the ratio of LH and FSH and local concentrations of estrogens and androgens. The action of LH and FSH on the follicle is strictly specialized: LH stimulates the process of de novo androgen synthesis by theca cells and has virtually no effect on granulosa cells, and FSH activates the aromatase system of the granulosa, which converts androgens synthesized in the theca into estradiol (15).
Estrogens and FSH inhibit atresia of the preantral follicle and stimulate the proliferation of granulosa cells, the synthesis of FSH receptors and the induction of LH receptors, starting at the periphery of the follicle and moving towards the center. The appearance of LH receptors in granulosa cells of large follicles is a prerequisite for the synthesis of progesterone by the corpus luteum. LH, through stimulation of androgen synthesis, limits and reduces the synthesis of receptors for FSH, LH, estradiol in follicle cells. The synergistic action of LH and FSH in the early follicular phase causes a significant increase in estrogen secretion by the ovary. This in turn induces an increase in the LH/FSH index, which shifts the synthesis of sex steroids in the follicles towards the preferential formation of androgens. In the normal course of events, by the 8th day of the menstrual cycle, the selection of the dominant follicle ends, the main property of which is the ability to enhance estrogen production in conditions of FSH deficiency and completely suppress the development of other follicles of the cohort with the help of intraovarian and hypothalamic-pituitary connections (8, 11, 18, 42) . If for any reason the dominant follicle dies, recruitment must occur again, since no other follicle in this cohort can take on the role of the dominant one. An important role in the process of suppression of other follicles is played by the polypeptide regulator inhibin, which selectively suppresses the secretion of FSH, and the follicle-regulating protein, which selectively suppresses the aromatase activity of granulosa.
On days 12–14 of the cycle, the dominant follicle is responsible for almost all the production of estradiol in large quantities, which causes a peak in LH and FSH, which is the cause of ovulation.
The FSH peak in the middle of the cycle is important for the normal functioning of the corpus luteum, ensuring the induction of the synthesis of LH receptors in the granulosa cells of the preovulatory follicle.
In healthy women, proper development of the dominant follicle causes:
- adequate production of estradiol, ensuring the maturation of the endometrium and the accumulation of progesterone receptors in its epithelium and the maturation of cervical mucus;
- full ovulation;
- preparation of receptors for LH in granulosa, which should turn into the corpus luteum.
Thus, the quality of the luteal phase is determined primarily by the processes occurring in the first phase of the cycle. According to the 1976 WHO classification, all disorders of the endocrine function of the ovaries are divided into 7 large groups:
- hypogonadotropic normoprolactinemic insufficiency;
- normogonadotropic normoprolactinemic insufficiency;
- hypergonadotropic insufficiency;
- anatomical form of amenorrhea;
- hyperprolactinemia;
- hyperprolactinemia;
- volumetric processes in the hypothalamic-pituitary region that do not change the secretion of prolactin (5).
The vast majority of patients with impaired ovarian function who seek treatment for infertility belong to the 2nd group of disorders according to the WHO classification - eugonadotropic hypothalamic-pituitary dysfunction. Clinically, in this group one can distinguish subgroup 2a - patients with spontaneous menstrual cycles - and subgroup 2b - patients with amenorrhea. Patients of subgroup 2a are characterized by insufficiency of the luteal phase due to impaired maturation of the dominant follicle, impaired ovulation and impaired function of the corpus luteum, as well as anovulatory menstrual cycles, characterized by the fact that the dominant follicle matures, but does not ovulate; during the period of atresia of the dominant follicle, luteinization of the granulosa and theca occurs , accompanied by sharply reduced progesterone production. In this case, the basal temperature either does not increase or increases slightly.
Luteal phase deficiency, anovulation and amenorrhea are usually an expression of the degree of endocrine disruption and often act as stages of one process (34).
The typical expression of type 2 ovarian failure is an increase in the LH/FSH ratio, accompanied by a small (compared to hormone-producing tumors) adrenal and/or ovarian hypersecretion of androgens (5). Traditionally, such forms of hypothalamic-pituitary-ovarian dysfunction were classified as polycystic ovary syndrome. However, this term is disputed by a number of authors. On the one hand, ovarian enlargement can occur with Cushing's syndrome, andrenogenital syndrome, with hormone-producing tumors, and sometimes in healthy adolescents. On the other hand, women with typical manifestations of this syndrome may have normal sized ovaries. In addition, with this syndrome, anatomical changes in the ovaries are only a consequence of disrupted hormonal interactions in the body.
Therefore, it is proposed to call this type of pathology hyperandrogenism syndrome with chronic anovulation (19). In terms of hormonal changes, the most characteristic signs are a LH/FSH ratio greater than 2 and an increase in the level of androgens (testosterone, androstenedione and DHEA-S) in the peripheral blood (17).
Compared with healthy women, in whom the main estrogen in the circulating blood is estradiol, women with hyperandrogenism syndrome have significantly increased estrone levels, which may exceed the concentration of estradiol. The main source of increased estrone levels in such patients is peripheral aromatization of androstenedione. Constant and monotonous production of estrone sensitizes the pituitary gland to the action of Gn-RH, resulting in an increase in the LH/FSH ratio secreted by the pituitary gland. In turn, high LH levels lead to excessive stimulation of the ovarian stroma and theca, resulting in excessive androgen production. Under these conditions, both the process of selection of the dominant follicle and its usefulness are sharply disrupted, which leads to opsomenorrhea, anovulation, luteal phase deficiency and amenorrhea (9, 27).
Hypothalamic-pituitary dysfunction in type II ovarian failure is a purely functional disorder in which positive feedback is disrupted. The etiology of hyperandrogenism syndrome with chronic anovulation is still unknown. It has been proven that heredity, central catecholamine disturbances, mental stress and obesity play an important role in the development of the syndrome (32).
Dysfunction of the adrenal cortex plays an important role in the development of the disease. In a significant proportion of patients, the adrenal glands are very sensitive to ACTH stimulation. In this regard, it has been hypothesized that the pituitary gland secretes a specific hormone that stimulates androgens of the adrenal cortex with a molecular weight of about 60,000 (32). Some patients are heterozygous carriers of the C-21-hydroxylase defect (38).
In addition, increased production of androgens by theca cells can also be caused by increased insulin levels due to the overlap of insulin specificity and local growth factors (3). Consequently, hirsutism and hyperandrogenism may be a manifestation of profound metabolic disorders.
Hyperandrogenic ovarian insufficiency is characterized by an increase in the amplitude and frequency of pituitary LH bursts (41).
An important role in pathogenesis is played by the influence of androgens on the level of testosterone and estrogen binding protein (TESB). With hyperandrogenism and obesity, the synthesis of TESH in the liver decreases, which leads to an increase in the active concentrations of estrogen and testosterone in the blood, as a result of which the manifestations of hyperandrogenism intensify. There are indications that non-hereditary intrauterine influences play an important role in the development of the syndrome, and that maternal hyperandrogenism may have an adverse effect on the maturation of various fetal enzyme systems (25). With hyperandrogenism syndrome, the ratio of norepinephrine and dopamine changes, and the resulting dopamine deficiency leads to increased LH release.
Impaired development of the dominant follicle and ovulation in normogonadotropic ovarian failure leads to the development of NLF (23).
There are 5 reasons for the development of NLF: impaired follicle maturation; insufficient stimulation of LH in the 2nd phase of the cycle; insufficient and/or delayed luteinization of the preovulatory follicle; mild forms of hyperprolactinemia; hyperandrogenism of various origins (23). The hormonal manifestation of NLF is a decrease in the production of progesterone by the corpus luteum, accompanied by normal or increased secretion of estradiol (relative hyperestrogenism). At the cellular level, NLF is manifested by increased cell divisions (endrometry, mammary gland, myometrium). Clinically, NLF manifests itself as premenstrual syndrome, menstrual irregularities, decreased fertility, benign breast tumors and uterine fibroids. The causes of infertility in NLF are insufficient endometrial maturity, which impedes normal implantation, and insufficient progesterone levels to support early pregnancy (26).
- The arcuate nucleus and the control of gonadotropin and prolactin secretion in the female rhesus monkey (Macaca mulatta). -Plant TM, Krey LS, Moossy J., McCormack JT, Hess DL, Knobil E. //Endocrinology, 1978, v. 102, N 1, p. 52-62.
- Baltzer J., Mickan H. Kern Gynäkologie. 4. Aufl. Stuttgart: Thieme, 1985. -685 S.
- Barbieri RL, Ryan KJ Hyperandrogenism, insulin resistance and acanthosis nigrans syndrome: A common endocrinopathy with distinct pathophysiological features. //American Journal of Obstetrics and Gynecology, 1983, v. 147, N 1, p. 90-101.
- Bohumil RJ Pulsatile variations in hormone levels. //Biorythms and human reproduction. — Ferin M., Halberg F., Richart RM, Van de Wiele RL (Eds) New York: Wiley, 1974, p. 107-131.
- Breckwoldt M. Störungen der Ovarialfunktion. //Reproductionsmedizin. -Bettendorf J., Breckwoldt M. (Hrsg.). Stuttgart; New York: Fisher, 1989, pp. 258-266.
- Central electrophysiological correlates of pulsatile luteinizing hormone secretion in the rhesus monkey. -Wilson RS, Kesner JS, Kaufman JM, Uemura T., Akema T., Knobil E. //Neuroendocrinology, 1984, v. 39, N 3, p. 256-260.
- Childs GV Functional ultrastructure of gonadotropes: a review. //Morphology of hypothalamus and its connections. -Ganten D., Pfaff D. (Eds.). Berlin: Springer, 1986, p. 49-98.
- Correlation of human follicular fluid inhibin activity with spontaneous an
- Davis OK, Ravnikar V. Induction of ovulation with Clomiphen Citrate. //Reproductive endocrine therapeutics. – Barbiery L., Schiff I. (Eds.). New York: AR Liss, Inc., 1988, p. 1-24.
- Diedrich K., Wildt L. Neue Wege in der Behandlung ovarieller Funktionsstorungen. Teil 1. //Neue Wege in der Diagnostik und Therapie der Weiblichen Sterilität. -Diedrich K., Hrsg. -Stuttgart: F. Enke, 1987, p. 26-40.
- DiZerega GG, Hodgen GD Folliculogenesis in the primate ovarian cycle. //Endocrine review 1981, v. 2, N 1, p. 27-49.
- The effect of morphine on the electrophysiological activity of the hypothalamic luteinizing hormone-releasing hormone pulse generator in the rhesus monkey. -Kesner JS, Kaufman G., Wilson RC, Kuroda G., Knobil E. //Neuroendocrinology, 1986, v. 43, N 6, p. 486-488.
- Electrophysiological manifestation of luteinizing hormone releasing hormone pulse generator activity in the rhesus monkey: influence of a adrenergic and dopaminergic blocking agents. -Kaufman JM, Kesner JS, Wilson RS, Knobil E. //Endocrinology, 1985, v. 116, N 4, p. 1327-1333.
- Everett JW Central neural control of reproductive functions of the adenohypophysis. //Physiology review, 1964, v. 44, p. 373-431.
- Falck B. Site of production of estrogens in rat ovary as studied by microtransplants. //Acta physiologica Scandinavica, 1959, v. 163, N 1, p. 1.
- Ferin M., van Vugt D., Wardlaw S. The hypothalamic control of the menstrual cycle and the role of endogenous opioid peptides. //Recent progress in hormone research, 1984, v. 40, p. 441-485.
- Givens JR, Andersen RN, Umstot ES Clinical findings and hormonal responses in patients with polycystic ovarian disease with normal versus elevated LH levels. //Obstetrics and gynecology, 1976, v. 47, N 4, p. 388-394.
- Hoffmann F. Untersuchunden über die hormonale Regulation der Follikelreifung im Zyklus der Frau. //Geburtshilfe und Frauerheilkunde, 1961, Bd. 21, S. 554-560.
- Infertility, contraception and reproductive endocrinology. Ed. by D. R. Michell, Jr.; V. Davaian, 2nd edition. - Oradell: Medical Economics Books, 1986. -IX, 688 p.
- Kleitmann N. Sleep and wakefulness. - Chicago: Chicago University Press, 1963. -250 p.
- Lakoski JM Cellular electrophysiologycal approaches to the central regulation of female reproductive aging. //Neural control of reproductive function. -JM Lakoski, JR Perez-Polo, DKRassin (Eds.). -New York: Liss, 1989, p. 209-220.
- Lavie P., Kripke DF Ultradian circa 1½ hour rhythms: A multioscillatory system. //Life sciences, 1981, v. 29, N 24, p. 2445-2450.
- Lobo RA Polycystic ovary syndrome. //Infertility, contraception and reproductive endocrinology. Ed. by D. R. Michell, Jr. and V. Davajan, 2nd edition. — Oradell: Medical Economics Books, 1986, p. 319-336.
- Leyendecker G., Wildt L., Plotz EJ Die hypothamische Ovarialinsuffizienz.//Gynäkologe, 1981, Bd. 14, N 2, S. 84-103.
- Lobo RA Polycystic ovary syndrome. //Infertility, contraception and reproductive endocrinology. Ed. by D. R. Michell, Jr. and V. Davajan, 2nd edition. — Oradell: Medical Economics Books, 1986, p. 319-336.
- Mauvais-Jarvis P., Kutten F. Insuffisance gonadotrope dissociée (anovulation et dysovulation)
- The microinvironment of the human antral follicle: Interrelationships among the steroid levels in human antral fluid, the population of granulosa cells and the status of the oocyte in vivo and in vitro. -McNatty KP, Smith DM, Makris A., Osathanonolh R., Ryan KJ //Journal of clinical endocrinology and metabolism, 1979, v. 49, N 6, p. 851-860.
- Miller BT Peptide modulation of luteinizing hormone releasing hormone secretion. //Neural control of reproductive function. -JM Lakoski, JR Perez-Polo, DK Rassin (Eds.). New York: ARLiss, Inc., 1989, p. 255-271.
- Mode of action of progesterone in the blockade of gonadotropin surges in the rhesus monkey. -Pohl CR, Richardson WD, Marshall G., Knobil E. //Endocrinology, 1982, v. 110, N 4, p. 1454-1455.
- The Neuro-immune-endocrine connection. - Cotman C., Brinton RE, Galaburda A., McEwen BC -New York: Raven Press, 1986. -150 p.
- Page RB Pituitary blood flow. //American journal of physiology, 1982, v. 243, N 6, p. 427-442.
- Parker LN, Odell WB Control of adrenal androgen secretion. //Endocrine review, 1980, v. 1, N 4, p. 392-410.
- Perez-Polo JR Introduction: Neuroimmune modulation of reproductive function. //Neural control of reproductive function. -JM Lakoski, JR Perez-Polo, DK Rassin (Eds.). -New York: AR Liss, 1989, p. 307-309.
- Plotz EJ Differentialdiagnose und Therapie ovarieller Funktionsstörungen: Richtlinien fur die Praxis. //Gynäkologe, 1981, Bd. 14, N 2, S. 145-148.
- The pulsatile pattern of gonadotropin secretion and follicular development during the menstrual cycle and in women with hypothalamic and hyperandrogenic amenorrhea. -Wildt L., Schwilden H., Werner G., Roll C., Brensing KA, Vuckhaus J., Böhr M., Leyendecker G. //Brain and pituitary peptides II. — G. Leyendecker, H. Stock, L. Wildt (Eds.). -Basel: Karger, 1983, p. 28-36
- Rushton WAH Peripheral coding in the nervous system. //Sensory communication. -W.A. Rosenblith (Ed.). -New York: Wiley, 1961, p. 20-30.
- Shapiro S. Compass on the 90-minutes sleep-dream cycle. //Sleep and dreaming. -Hartman E. (Ed.) -Boston: Little and Brown, 1970, p. 40-49.
- An update of congenital adrenal hyperplasia. — New MI, Dupont B., Pang S., Pollack M., Levine SL // Recent progress in hormone research, 1981, v. 37, p. 105-181.
- Wildt L. Die endokrine Kontrolle der Ovarialfunktion und die Pathologie endokriner Ovarialfunktionsstörungen. // Neue Wege in Diagnostik und Therapie der weiblichen Sterilität. -Hrsg. von K. Diedrich. - Stuttgart: Enke, 1987, S. 1-25.
- Wildt L. Hypothalamus. //Reproductionsmedizin. —Hrsg. von Bettendorf G., Breckwoldt M. - Stuttgart: Fischer, 1989, S. 6-22.
- Yen SSC The polycystic ovary syndrome. //Clinical endocrinology, 1980, v. 12, N 2, p. 177-207.
- Zeleznik AJ, Schuler HM, Reichert LC, Jr. Gonadotropin binding sites in the rhesus monkey ovary: Role of the vasculature in the selective distribution of human chorionic gonadotropin to the preovulatory follicle. //Endocrinology, 1981, v. 109, N 2, p. 356-362.
d induced follicular maturation. -Murrs RP, Lobo JD, Campeau JD, Nakamura RM, Brown J., Ujita EL, DiZerega GS //Journal of Clinical Endocrinology and Metabolism, 1987, v. 64, N 1, p. 148-152.
. //Medecine de la reproduction. Gynécologie endocrinienne. -Paris: Flammarion, 1982, p. 305-319.
.
Tags: menstrual cycle
Hypothalamus - pituitary gland - thyroid gland
The release of thyroid hormones is controlled by two “superior” endocrine glands. The area of the brain that connects the nervous and endocrine systems is called the hypothalamus. The hypothalamus receives information about the level of thyroid hormones and secretes substances that affect the pituitary gland. The pituitary gland is also located in the brain in the area of a special depression - the sella turcica. It secretes several dozen hormones that are complex in structure and action, but only one of them acts on the thyroid gland - thyroid-stimulating hormone or TSH.
The level of thyroid hormones in the blood and signals from the hypothalamus stimulate or inhibit the release of TSH. For example, if the amount of thyroxine in the blood is small, then both the pituitary gland and hypothalamus will know about it. The pituitary gland will immediately release TSH, which activates the release of hormones from the thyroid gland.
Low temperature and stress lead to more active release of TSH and, accordingly, an increase in the amount of T3 and T4 in the blood. During sleep, TSH is practically not formed.
The triple axis of interaction between the hypothalamic-pituitary-thyroid system allows not only the higher glands to know about the amount of thyroid hormones, but also the nervous system, which directly depends on and influences hormonal regulation.
Pituitary hormones
Melanocyte stimulating hormone (MSH)
Area of effect: Skin.
Functions: Stimulates the production of melanocytes, which affect skin color.
Antidiuretic hormone, or vasopressin (ADH)
Area of action: Kidneys.
Functions: Retains water in the kidneys, regulates blood pressure.
Growth hormone, or somatotropin (GH, STH or RG)
Area of action: Whole body.
Functions: Stimulates the growth of muscles, bones and organs during childhood and puberty.
Thyrotropin (TSH)
Area of action: Thyroid gland.
Functions: Stimulates the activity of the thyroid gland.
Oxytocin
Area of effect: Uterus
Functions: Provokes uterine contractions during childbirth.
Adrenocorticotropin (ACTH)
Area of action: Adrenal glands.
Functions: Stimulates the production of corticosteroids by the adrenal glands.
Prolactin (LTG)
Area of effect: Chest.
Functions: Provokes the production of milk by the mammary glands after childbirth.
Action of thyroid hormones
Unlike most hormones, which act only on certain target cells (for example, for estradiol these are the genitals), thyroid hormones are necessary for normal functioning of all tissues without exception. Penetrating into the cell, the hormone is sent to the nucleus, where it binds to certain areas on the chromosomes and stimulates a complex of reactions, which leads to the activation of oxidation and reduction processes.
The effect of thyroid hormones on the body:
- increased heat release
- activation of protein synthesis necessary for the construction of new cells
- proper growth and development of the central nervous system, especially the brain (especially important for children)
- strengthening the processes of reabsorption in the intestine, the formation of glucose from proteins and fats, increasing the level of glucose in the blood
- stimulation of fat breakdown in fat depots, which leads to weight loss
- anabolic effect – body growth, maturation, bone differentiation
- red blood cell formation
- normal development of the genital organs and the release of sex hormones.
How many hormones should there be?
There must be enough hormones to ensure normal functioning of the body. Tests can accurately determine the level of thyroid hormones. The “gold standard” among laboratory methods for analyzing thyroid hormones is radioimmunoassay. But due to the difficulties of using radioactive isotopes, most laboratories conduct tests using the ELISA (enzyme-linked immunosorbent assay) method.
The amount of thyroid hormones depends on:
— the intensity of signals coming from the brain and regulating the functioning and level of thyroid hormones
- the number of working cells in the gland itself
- the presence of a sufficient amount of iodine, which is necessary for the synthesis of hormones.
Normal thyroid hormone levels:
— total triiodothyronine T3 — 1.2-2.8 mIU/l
— total thyroxine T4 — 60.0-160.0 nmol/l
— free triiodothyronine FT3 — 2.5 — 5.8 pmol/l
— free thyroxine FT4 — 11.5-23.0 pmol/l
— thyroid-stimulating hormone TSH, TSH — 0.17-4.05 mIU/l
— thyroglobulin Tg — less than 50 ng/ml
When performing tests for thyroid hormones, not only their quantity is assessed, but also antibody levels. When there are disturbances in the immune response system, antibodies begin to form not only against foreign organisms, but also against “native” tissues. Some of these antibodies disrupt the functioning of the thyroid gland and the action of its hormones. The most common are TSH receptor Ab, anti-thyroglobulin antibody (ATG) and anti-thyroid peroxidase antibody (anti-ATPO).
Antibodies to TSH receptors are similar in structure to TSH and their attachment to receptors on the thyroid gland leads to the active release of T3 and T4. Antibodies to thyroglobulin appear in autoimmune Hashimoto's thyroiditis and pregnancy. Monitoring their levels in the blood indicates the activity of inflammation. Anti-ATPO - antibodies to thyroperoxidase (AMC, antibodies to the microsomal fraction) lead to the destruction of the gland and the release of hormones into the blood.
A condition in which there is enough thyroid hormone for the body is called euthyroidism.
Treatment methods used
Standard treatment for pituitary adenoma consists of drug, radiation and neurosurgical therapies. The optimal method of exposure is determined based on the diagnostic results: it depends on the patient’s condition, the characteristics of the tumor itself, and the presence of concomitant diseases.
Drug therapy
Drug therapy is prescribed in 90% of cases. The drugs help get rid of the symptoms of the disease and improve the patient’s well-being. Initially, vitamin and restorative complexes are prescribed. Next, you need to determine the type of tumor:
- for prolactinomas, dopamine agonists or ergoline drugs are prescribed,
- for somatotropinomas - somatostatin agonists,
- for corticotropinoma - steroidogenesis blockers.
If necessary, hormone replacement therapy is performed. If the chosen treatment regimen does not bring results for a long time, then a more radical method of treatment is selected: radiation therapy or surgery.
Radiation therapy
For microadenomas, proton, gamma and remote therapy are used. The essence of the tactic is the introduction of a radioactive substance directly into the pathological area. Thanks to this effect, it is possible to stop the growth of the tumor and achieve regression. Typically, radiation therapy is performed when surgery is not possible and the patient refuses surgery. After such exposure, the pituitary adenoma gradually decreases in size and can be completely destroyed.
Surgical intervention
If the tumor is large and associated complications develop (hemorrhages, cyst formation, visual impairment), its removal using the transcranial method is indicated. The operation involves craniotomy. In some cases, intervention may be performed using endoscopic techniques.
The choice of specific treatment tactics mainly depends on the type of tumor. For prolactinoma, radiation exposure is practically useless, while for corticotropinoma it is highly effective. If the tumor does not cause any disturbances in the body and the patient does not complain of deterioration in health, doctors choose a wait-and-see approach.
Not enough thyroid hormones
Reduced function of the thyroid gland - hypothyroidism, occurs when there is a deficiency of iodine or the intake of substances that disrupt the formation of hormones. Rarer causes of hypothyroidism are the use of certain drugs (for example, cordarone), removal of the gland as a result of tumors or deficiency of TSH secretion. Hypothyroidism in childhood leads to growth retardation, disproportionate growth, mental retardation, and cretinism. Hypothyroidism in adults is called myxedema.
Manifestations of thyroid hormone deficiency:
- weight gain that is not reduced by diet and exercise
- general weakness, constant fatigue, fatigue
- constantly depressed mood
- menstrual irregularities, infertility
– low body temperature (35.6-36.3ºС)
- dry, swollen skin, itching, dandruff that does not disappear when using medicated shampoos, changes in nails
- constant constipation
- constant swelling of the legs, feet, puffiness of the face
- low blood pressure, low heart rate
- inability to warm up even in a warm room
- pain in muscles and joints
- deterioration of memory and reaction speed
One of the forms of hypothyroidism is endemic goiter, which develops when there is insufficient iodine intake in the body. This situation is typical for areas where its level in water and soil is low. Switzerland was one of the first countries to introduce mandatory iodization of salt, sunflower oil and bread back in 1922. There are no cases of hypothyroidism in Switzerland today. Areas of iodine deficiency in Russia are the North Caucasus, the Urals, Altai, the Siberian Plateau, the Far East, the Upper and Middle Volga region, in the North and Central regions of the European part of the country. In Ukraine, these are Volyn, Transcarpathian, Ivano-Frankivsk, Lviv, Rivne, Ternopil regions.
During accidents at nuclear power plants, large amounts of radioactive iodine are released into the air. Radioactive iodine can irradiate the gland from the inside and integrate into thyroid hormones, which leads to active tumor growth. Carrying out iodine prophylaxis helps prevent the entry of radiation iodine into the thyroid gland by replacing it with a stable isotope.
The role of dopaminomimetics in the treatment and prevention of relapses of pituitary adenoma
The pituitary gland and hypothalamus are functionally a single whole. The hypothalamus is part of the diencephalon, and the pituitary gland develops from two ectodermal primordia of different origins: the protrusion of the primary oral recess (Rathke's pouch) and the protrusion of the floor of the third ventricle of the brain (the infundibulum). The pituitary gland, lower cerebral appendage, or pituitary gland (hypophysis cerebri, glandula pituitaris), is a complex endocrine organ located at the base of the skull in the sella turcica of the main bone and anatomically connected by a pedicle to the bottom of the third cerebral ventricle of the diencephalon. It consists of three lobes: the anterior lobe, the middle lobe and the posterior lobe. The anterior and middle lobes are combined under the name adenohypophysis, and the posterior lobe is called the neurohypophysis. The neurohypophysis also includes the median eminence (medial eminence, located on the border between the adenohypophysis and the hypothalamus of the diencephalon).
The bed of the pituitary gland, the sella turcica, like the pituitary gland, has an oval shape. It is lined with the dura mater, between the layers of which the pituitary gland is located. The entrance to the sella turcica is covered by a sheet of dura mater, which is called the diaphragm of the sella turcica. The pituitary stalk passes through the hole in the diaphragm. Normally, the arachnoid membrane is located on the upper surface of the diaphragm of the sella turcica and does not descend into its cavity. In the presence of congenital defects of the diaphragm of the sella turcica, the arachnoid membrane extends into the cavity of the sella turcica, allowing cerebrospinal fluid to penetrate here, which leads to the development of empty sella syndrome.
The anterior lobe of the pituitary gland produces protein hormones (growth hormone (GH) and prolactin), glycoproteins (follicle-stimulating, luteinizing and thyroid-stimulating hormone (TSH)), as well as adrenocorticotropic hormone (ACTH), endorphins, lipotropins and melanocyte-stimulating hormones. The posterior lobe of the pituitary gland serves as a reservoir for storing neurohormones - vasopressin and oxytocin, which enter here along the axons of neurons located in the hypothalamic nuclei.
The causes of deficiency of pituitary hormones can be: defects in blood supply, hemorrhage, congenital underdevelopment of the pituitary gland, meningitis or encephalitis, compression of the pituitary gland by a tumor, traumatic brain injury, exposure to certain drugs, radiation, surgery.
A lack of pituitary hormones can lead to a secondary deficiency of hormones in other endocrine glands (secondary hypothyroidism, diabetes insipidus), as well as to severe physical disorders (pituitary dwarfism, hypopituitarism).
The cause of excess pituitary hormones in most cases is a tumor of the pituitary gland itself - an adenoma. At the same time, the level of those hormones that are produced by adenoma cells increases, while the level of all other hormones can significantly decrease due to compression of the remaining part of the pituitary gland.
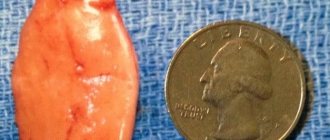
Among all tumors affecting the pituitary gland, adenoma ranks first. Typically, pituitary adenoma occurs in adults, but sometimes the tumor is found in childhood [1]. Half of all cases of the disease occur at an average age of 30–50 years, with equal frequency for men and women, accounting for up to 15% of all intracranial neoplasms [2]. The true prevalence of this tumor is difficult to establish, since many of them exist asymptomatically for a long time. The detection rate is only 2 people per 100,000 population. At autopsy, pituitary adenomas are found in 10–20% of patients who die from diseases unrelated to the pituitary gland [3].
Until recently, pituitary adenomas were divided into acidophilic (eosinophilic), accompanied by hypersecretion of GH (acromegaly or gigantism), basophilic, secreting ACTH, as well as chromophobic, occurring without disruption of hormone secretion, and mixed. However, in many cases there was no sufficient correlation between the clinical picture of the disease and the histological structure of the pituitary adenoma. However, in 1995, E. Horvath and K. Kovacs, using histological and other types of studies when studying 1700 pituitary adenomas, proposed a modified classification taking into account the frequency of occurrence of different types of adenomas. According to their classification, there are somatotrophic, lactotrophic, mammosomatotrophic, corticotrophic, thyrotrophic, gonadotrophic, plurihormonal, “silent” and other types of adenoma.
Pituitary adenoma (adenoma hypophysis) is a benign tumor arising from the glandular cells of the anterior pituitary gland (adenohypophysis) and localized in the cavity of the sella turcica of the sphenoid bone of the base of the skull (Fig. 1).
The very concept of “pituitary adenoma” is collective and includes a whole group of tumors that cause diseases of various manifestations. Like all tumors, pituitary adenomas are divided by size, direction of growth, histological features, and also by hormonal activity. The modern classification of pituitary adenomas is based on a comparison of clinical symptoms and the concentration of tropic hormones in the blood with the immunohistochemical and electron microscopic characteristics of the tumor.
There are several types of classifications of pituitary adenomas. One of them is based on the hormonal activity of tumors, which has been actively used since the early 70s of the 20th century. According to this classification, adenomas are divided into hormonally inactive (25–30%) and hormonally active (70–75%) [4].
Pituitary adenomas that occur without clinical manifestations of hypersecretion of pituitary hormones are called “inactive” pituitary adenomas, the growth of which leads to a decrease in the function of the pituitary gland - hypopituitarism. Until the onset of neurological symptoms, such as headache, blurred vision, associated with the impact of a large tumor on the surrounding structures, hormonally inactive pituitary adenomas are clinically “silent” or “silent” tumors. The terms “clinically non-functioning” adenomas are also used in the literature [5]. However, there are also hormonally active adenomas, which include ACTH-producing, prolactin-secreting, GH-producing, TSH-producing, as well as gonadotropic adenomas. The frequency of occurrence of somatotropin is 20–25%, prolactin - 40%, corticotropin - 7%, thyrotropin - 3% of the total number of pituitary adenomas. Mixed tumors—prolactosomatotropinomas and prolactocorticotropinomas—are relatively uncommon. The remaining types of pituitary adenomas are rare tumors.
In addition, there is a classification of pituitary adenomas according to the direction of growth. The growth pattern of a pituitary adenoma is determined by the relationship of the tumor to the sella turcica, in which the normal pituitary gland is located, and to the surrounding structures. At an early stage, pituitary adenomas develop in the cavity of the sella turcica (endosellar tumors). Gradually increasing, the tumor can spread downward, into the sphenoid sinus (infrasellar), upward - towards the diaphragm of the sella turcica and the optic chiasm (suprasellar), laterally, affecting the structures of the cavernous sinus, the basal parts of the temporal lobes of the brain and the great vessels of the head, posteriorly - in the direction of the brain stem (retrosellar) and anteriorly - in the direction of the frontal lobes, orbit, ethmoidal labyrinth and nasal cavity (antesellar). Very often, the direction of growth of a pituitary adenoma is different (up, to the side, down) - then the tumor is called endo/supra/infra/laterosellar. Based on their size, pituitary tumors are divided into microadenomas (less than 1 cm in diameter) and macroadenomas (diameter more than 1 cm).
The reasons for the development of pituitary adenomas have not yet been fully elucidated, although it is known that some of them may be genetically determined. Factors predisposing to the development of pituitary tumors include neuroinfections, chronic sinusitis, traumatic brain injury, hormonal imbalance, and adverse intrauterine effects on the fetus. Recently, the etiological role has been partially assigned to long-term use of oral contraceptives.
The development of a pituitary tumor is a multistage process that involves, along with somatic mutations in pituitary cells, many other additional factors - hormonal, autocrine and paracrine. Important pathogenetic factors involved in tumorigenesis in the pituitary gland are hypothalamic hormones, neurotransmitters and growth factors [6]. At the same time, it should be emphasized that disorders of hypothalamic regulation and other specified factors, unlike oncogenic mutations, only contribute to the development of a pituitary tumor, but are not its direct cause [7].
There is the concept of a primary lesion of the hypothalamus with secondary involvement of adenohypophysis tissue in the process [8], as well as the concept of a primary lesion of the pituitary gland, which results in the occurrence of an adenoma [9]. The formation of some forms of pituitary adenomas (thyrotropin, gonadotropin) against the background of a primary decrease in the activity of peripheral endocrine glands (with primary hypothyroidism, hypogonadism) occurs due to hyperstimulation of the pituitary gland by hypothalamic releasing hormones. This indicates the existence of different mechanisms for the formation of pituitary adenoma.
It has been proven that pituitary cells are capable of producing various growth factors, including basic fibroblast growth factor, which has powerful mitogenic and angiogenic potential, and have corresponding receptors [3].
The clinical picture of pituitary adenomas is polymorphic and is represented by various groups of symptoms, the appearance of which is determined by the functions of hormones secreted by one or another form of the tumor (
).
Clinical manifestations of hormonally active pituitary adenomas consist of endocrine-metabolic syndrome, ophthalmic-neurological and radiological symptoms. The severity of endocrine metabolic syndrome reflects the level of excessively produced pituitary hormone and the degree of damage to the tissue surrounding the tumor.
In some pituitary adenomas (corticotropinomas, some thyrotropinomas), the clinical picture is caused not so much by the excessive production of the tropic hormone itself, but by the associated activation of the target organ, expressed by hypercortisolism and thyrotoxicosis. Ophthalmic and neurological symptoms indicating the presence of a pituitary adenoma (primary atrophy of the optic nerves, changes in the visual field such as bitemporal hemianopsia, hypoxia, etc.) depend on the suprasellar growth of the tumor. Due to the pressure of the tumor on the diaphragm of the sella turcica, headache occurs, which is usually localized in the frontal, temporal and retroorbital regions. This pain is usually dull in nature, is not accompanied by nausea, does not depend on body position, and is not always relieved by painkillers. Further upward growth of the tumor leads to damage to the hypothalamic structures. The growth of pituitary adenoma in the lateral direction causes compression of the III, IV, VI and branches of the V cranial nerves with the development of ophthalmoplegia and diplopia. The growth of the tumor downward, towards the bottom of the sella turcica, and the spread of the process into the sinus of the sphenoid bone and ethmoid sinuses may be accompanied by a feeling of nasal congestion and liquorrhea [17].
A sudden increase in headache and ophthalmic neurological symptoms in patients with pituitary adenoma is most often associated with either accelerated tumor growth, for example, during pregnancy, or with hemorrhage into the tumor. Hemorrhage into the tumor is considered a serious but not fatal complication. Hemorrhages into the pituitary adenoma occur quite often and can lead, in addition to increased headaches, visual disturbances and the development of hypopituitarism, to spontaneous “cure” from a hormonally active pituitary adenoma. Spontaneous “cures” are most common in prolactinomas. The increase in tumor during pregnancy is possibly due to the inevitable increase in the adenohypophysis during this period; It was noted that in most patients with prolactinomas, the tumor decreases after delivery [18].
Symptoms of pituitary adenoma, revealed by X-ray examination, are changes in the shape and size of the sella turcica, thinning and destruction of the bone structures that form it, etc. With computed tomography, the tumor itself can be visualized.
Certain hormonally active pituitary adenomas are characterized by specific clinical symptoms. Prolactinomas in women manifest as galactorrhea-amenorrhea syndrome. Often the main endocrine manifestation of these tumors is only galactorrhea, or only menstrual irregularities, or infertility, but more often a combination of these symptoms is noted.
About a third of women with prolactinomas experience moderate obesity, mild hypertrichosis, acne, seborrhea of the scalp, sexual dysfunction - decreased libido, anorgasmia, etc. In men, the main endocrine manifestations of prolactinoma are sexual dysfunction (decreased libido, impotence), gynecomastia and galactorrhea are relatively rare. In women with prolactinomas, by the time the tumor is detected, ophthalmic neurological disorders occur in no more than 26% of cases; in men, ophthalmic neurological symptoms dominate. This is apparently due to the fact that in women prolactinomas are more often detected at the microadenoma stage, and in men, due to the slow increase in nonspecific symptoms such as sexual weakness, etc., a tumor of a larger size is almost always detected [19] .
Somatotropinomas are clinically manifested by acromegaly syndrome or gigantism in children. With acromegaly, in addition to the skeletal and soft tissue changes typical of this disease, blood pressure may increase, obesity and symptoms of diabetes may develop. Enlargement of the thyroid gland is often observed, often without dysfunction. Hirsutism, the appearance of papillomas, nevi, warts on the skin, severe greasiness of the skin, and increased sweating are often noted; the performance of patients is reduced. Ophthalmoneurological symptoms with somatotropinomas develop at a certain stage with extrasellar tumor growth. In addition to the symptoms listed above, peripheral polyneuropathy is noted, manifested by paresthesia, decreased sensitivity in the distal extremities, and pain in the extremities [20].
Diagnosis of pituitary tumors comes down to examination by specialists (neurosurgeon, endocrinologist, ophthalmologist), as well as radiography of the skull, hormonal blood tests, computed tomography of the brain and magnetic resonance imaging. Diagnosis of pituitary adenoma must be comprehensive. The pronounced emotional lability of patients with a pituitary adenoma, the difficulties of a diagnostic search, the likelihood of overdiagnosis, the slow growth and benign clinical course of many pituitary adenomas require tactful and careful familiarization of patients with the results of the examination.
Differential diagnosis is carried out with hormonally inactive tumors located in the area of the sella turcica, with tumors of non-pituitary localization that produce peptide hormones, and with hypothalamic-pituitary insufficiency of non-tumor origin. It is necessary to differentiate pituitary adenoma from empty sella syndrome, which is also characterized by the development of ophthalmic neurological syndrome.
In addition, it is necessary to prove that the endocrine metabolic syndrome was not the result of taking certain medications or neuro-reflex effects. Thus, neuroleptics, a number of antidepressants and antiulcer drugs can cause the development of galactorrhea, and corticosteroids contribute to the appearance of Cushingoidism. Frequent self-palpation of the mammary glands, the presence of an intrauterine contraceptive, and chronic adnexitis cause the occurrence of reflex galactorrhea.
To identify an abnormal response of adenomatous tissue to pharmacological effects, special stress pharmacological tests are also used. If a pituitary adenoma is suspected, the patient should be referred for consultation to an ophthalmologist. The study of visual acuity and fields, examination of the fundus allows us to diagnose visual disturbances (chiasmal syndrome), sometimes damage to the oculomotor nerve.
Today, there are three main types of treatment for patients with pituitary adenomas: neurosurgical (transsphenoidal, transcranial tumor removal), radiation (proton therapy, gamma therapy, gamma knife) and medication. Among the latter, dopamine agonists (bromocriptine, cabergoline, levodopa, quinagolide), somatostatin analogues (lanreotide, octreotide) and somatotropin receptor blockers are distinguished.
The choice of treatment for pituitary adenoma depends on the type of tumor (hormonally inactive or hormonally active), its size, severity and severity of clinical manifestations. The effectiveness of surgical treatment, external and interstitial radiation therapy, as well as drug treatment depends on the stage of tumor development and the severity of clinical symptoms (
).
Prolactinomas, regardless of size, in the absence of increasing visual impairment, are first treated conservatively with dopamine receptor agonists, and with long-term treatment, women can be allowed to become pregnant. Treatment of endosellar prolactinomas refractory to drug therapy is surgical. Precision proton irradiation is also used. Preference is given to microsurgical treatment methods. For large tumors extending to parasellar structures, neurosurgery is performed followed by postoperative radiation therapy [21].
For somatotropin and prolactosomatotropin in endosellar tumor localization, surgical treatment and proton radiation therapy are alternative methods. If radical surgical treatment is impossible due to tumor growth into the ethmoid sinuses and orbit, or if the tumor is extremely large in the postoperative period, remote gamma therapy is performed to prevent tumor growth, and dopamine receptor agonists are used.
Corticotropinomas in young patients, manifested by Nelson's syndrome or Itsenko-Cushing's disease of mild or moderate severity, are more often subjected to external beam radiation therapy. For small tumors, preference is given to proton irradiation. In severe cases, the goal of the first stage of treatment is to eliminate or reduce the degree of hypercortisolism using chemotherapy and surgical removal of one or both adrenal glands, and external beam irradiation of the pituitary gland, preferably proton, is carried out in the next stage of treatment [21].
Thyrotropinomas and gonadotropinomas are treated depending on their size and prevalence, starting with hormone replacement therapy. In the future, if necessary, surgical treatment and radiation therapy are added. To treat hormonally inactive pituitary adenomas, a complex approach is used (surgical treatment and radiation therapy), and subsequently patients are prescribed corrective hormone therapy [21].
The essence of drug treatment for pituitary adenoma is to reduce the effect of hormones produced by the tumor. However, long-term (often lifelong) drug treatment is not effective in all patients, and in addition, it is inappropriate in cases where the tumor size is quite large. Most often, drug treatment is used at the stages of preparation for surgery and in the postoperative period. The range of drugs for the treatment of pituitary adenoma is presented in table. 3.
There is no doubt about the appropriateness of a pathogenetic approach to solving the problem of treating pituitary adenoma, and therefore the group of dopaminomimetics (D2 dopamine receptor agonists) deserves special attention. They significantly reduce the level of hormones produced by the tumor, while simultaneously reducing the size of the tumor [22]. Dopaminomimetics became known in 1972, when the effectiveness of bromocriptine was shown in the treatment of patients with pituitary adenoma that simultaneously secretes GH and prolactin. However, in recent years, new generation dopamine agonists—quinagolide and cabergoline—have been used in the treatment of hormonally active pituitary adenomas. Moreover, the latter drug is now the drug of choice for the treatment of pituitary adenoma (Physicians Desk Reference, 2005) [10].
Cabergoline is able to reduce prolactin levels, restore sexual function and slow tumor growth in most patients, with a minimum of side effects [23]. Thus, in a 4-week, double-blind, placebo-controlled study, 900 patients with hyperprolactinemia caused by a hormonally active pituitary adenoma received cabergoline in fixed doses of 0.125, 0.5, 0.75 and 1.0 mg twice a week [24 ]. The severity of most side effects was mild or moderate. The range of adverse events that occurred while taking cabergoline is presented in Fig. 2.
In these patients, additional adverse events such as hallucinations, confusion, and peripheral edema were identified. Rarely, heart failure, pleural effusion, pulmonary fibrosis, gastric or duodenal ulcers have been reported, and one case of constrictive pericarditis has been reported. This is why periodic echocardiography is necessary in patients receiving long-term treatment with cabergoline.
A number of studies have established a connection between increased prolactin levels and deterioration of spermogram parameters [15, 16]. Treatment of hyperprolactinemia caused by pituitary adenoma with cabergoline has a positive effect on sperm parameters [11, 12]. When analyzing studies of sperm recovery after treatment with various dopaminomimetics (cabergoline, quinagolide or bromocriptine), more significant positive changes in sperm count, motility, forward movement and normalization of sperm morphology were noted in the cabergoline group [13, 14].
Thus, the evidence base of dopaminomimetics, their high efficiency and wide safety profile make it possible to prescribe drugs containing cabergoline as part of complex treatment and prevention of relapses of pituitary adenoma.
Literature
- Wilson TM, Yu-Lee LY, Kelley MR Coordinate gene expression of luteinizing hormone-releasing hormone (LHRH) and the LHRH-receptor after prolactin stimulation in the rat Nb2 T-cell line: implications for a role in immunomodulation and cell cycle gene expression // Mol Endocrinol. 1995; 9:44–53.
- Kushel Yu. V. Hormonally inactive pituitary adenomas // Problems of endocrinology. 1993, no. 1.
- Thapar K., Kovacs K., Laws ER Pituitary Adenomas: current concepts in classification, histopathology and molecular biology // The Endocrinologist. 1993, 3(1): 39–57.
- Katznelson U., Alexander JM, Klibanski AJ Clinical review 45: clinically nonfunctioning pituitary adenomas // Clin Endocrinol Metab. 1993; 76:5:1089–1094.
- Molitch ME Clinical review 65. Evaluation and treatment of the patient with a pituitary incidentaloma // J Clin Endocrinol Metab. 1995; 80:1:3–6.
- Thapar K., Stefaneanu L., Kovacs K., Scheithauer BW, Lloyd RV, Muller PJ, Laws ER Jr. Estrogen receptor gene expression in craniopharyngiomas: an in situ hybridization study // Neurosurgery. 1994; 35(6):1012–1017.
- Reichlin S. In: Faglia G., Beck-Peccoz P., Ambrosi B., Travaglini P., Spada A. Pituitary adenomas: new trends in basic and clinical research // Elsevier. 1991; 113–121.
- Asa SL, Kovacs K., Stefaneanu L. Pituitary adenomas in mice transgenic for growth hormone-releasing hormone // Endocrinology. 1992; 131:2083–2089.
- Ezzat S., Melmed S. The role of growth factors in the pituitary // J Endocrinol Invest. 1990, 13: 691–698.
- 57th Physicians' Desk Reference, 2005, p. 2740–2742.
- Merino G., Carranza-Lira S., Martinez-Chequer JC Hyperprolactinemia in men with asthenozoospermia, oligozoospermia, or azoospermia // Arch Androl. 1997, 38(3): 201–206.
- Saie DJ Hyperprolactinemia presenting with encephalomalacia-associated seizure disorder and infertility: a novel application for bromocriptine therapy in reproductive endocrinology // Neuro Endocrinol Lett. 2005; 26(5):533–535.
- De Rosa M., Colao A., Di Sarno A., Ferone D., Landi ML, Zarrilli S. Cabergoline treatment rapidly improves gonadal function in hyperprolactinemic males: a comparison with bromocriptine // Eur J Endocrinol. 1998, 138(3):286–293.
- De Rosa M., Ciccarelli A., Zarrilli S. The treatment with cabergoline for 24 months normalizes the quality of seminal fluid in hyperprolactinaemic males // Clin Endocrinol. 2006; 64(3):307–313.
- Vandekerckhove P., Lilford R., Vail A. Androgens versus placebo or no treatment for idiopathic oligo/asthenospermia // Cochrane Database Syst Rev. 2000; 2: CD00015.
- Ciccarelli A., Guerra E., De Rosa M., Milone F., Zarrilli S., Lombardi G., Colao A. PRL secreting adenomas in male patients // Pituitary. 2005; 8 (1): 39–42.
- Kasumova S. Yu. Functional morphology of pituitary adenomas. dis. Doctor of Medical Sciences M., 1985, p. 360.
- Dedov I. I., Melnichenko G. A. Persistent galactorrhea-amenorrhea. M., 1985; With. 166–180.
- Vaks V.V., Kadashev S.Yu., Kasumova S.Yu. Long-term results of postoperative treatment for “inactive” pituitary adenomas // Problems of endocrinology. 2001, No. 1, p. 16–19.
- Abdel Gadir A., Khatim MS, Mowafi RS, Alnaser HMI, Alzaid HGN, Shaw RW Hormonal changes in patients with polycystic ovarian disease after ovarian electrocautery or pituitary desensitization // Clin Endocrinol. 1990, 32: 749–754.
- Kovacs K., Black PM, Zervas NT, Ridgway EC Secretory tumors of the pituitary gland // Raven Press, New York, 1985; 365–376.
- Kulakov V.I., Serov V.N. Rational pharmacotherapy. M., 2005, p. 587.
- Dos Santos Nunes V., El Dib R., Boguszewski CL, Nogueira CR Cabergoline versus bromocriptine in the treatment of hyperprolactinemia: a systematic review of randomized controlled trials and meta-analysis // Pituitary. 2011.
- Shmakov R.G., Emelyanova A.I., Polushkina E.S. Modern aspects of lactation suppression // Attending Physician. 2009, No. 11, p. 24–28.
A. S. Skotnikov M. A. Feldman MGMSU , Moscow
Contact information for authors for correspondence
Excess thyroid hormones
With hyperthyroidism - increased work of the thyroid gland, increased synthesis and secretion of T3 and T4, an increase in the size of the gland, exophthalmos (bulging eyes).
Symptoms of elevated thyroid hormone levels:
- - weight loss with increased appetite
- - general weakness, fatigue
- - permanent excitement
- - menstrual irregularities, infertility
- - increased body temperature, sometimes at certain hours (36.9-37.5ºС)
- - dry and sagging skin
- - increased heart rate and high blood pressure
- - feelings of heat
- - deterioration of memory and reaction speed
Hyperthyroidism is observed in the following diseases of the thyroid gland: Bazedow-Graves disease (diffuse toxic goiter), Plummer disease (nodular toxic goiter), de Quervain's viral thyroiditis, autoimmune Hashimoto's thyroiditis. More rare causes of an increase in the amount of thyroid hormones are excessive consumption of thyroid hormones for treatment (thyroxine, euthyrox) or for the purpose of losing weight, for tumors of the ovaries and pituitary gland, and an overdose of iodine preparations.
What to do?
In order to determine the quality of the thyroid gland, you need to take tests for hormones and antibodies, as well as do an ultrasound examination. The most important hormones in the thyroid gland are the assessment of free T4 and TSH levels. An ultrasound will show the structure of the gland, its size and volume, and will identify nodes and cysts.
To prevent thyroid diseases, it is worth ensuring that you get enough iodine and tyrosine from your diet. Iodine is found in iodized salt and sunflower oil, kelp seaweed, fish (herring, flounder, cod, halibut, tuna, salmon), crabs, shrimp, squid and other seafood, feijoa. Sources of tyrosine are milk, peas, eggs, peanuts, beans. A nutritious and balanced diet ensures the balance of thyroid hormones and prevents thyroid disease.
Be healthy!