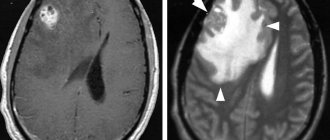
A cerebral pituitary adenoma (PPA) is a tumor of the glandular tissue of the brain appendage. The pituitary gland is a significant endocrine gland in the human body, located in the lower part of the brain in the pituitary fossa of the sella turcica. This small organ of the endocrine system, weighing only 0.7 g in an adult, is responsible for its own production of hormones and control over the synthesis of hormones by the thyroid and parathyroid glands, and the genitourinary organs. The pituitary gland is involved in the regulation of water-fat metabolism, is responsible for a person’s height and weight, the development and functioning of internal organs, the onset of labor and lactation, the formation of the reproductive system, etc. It is not for nothing that doctors call this gland a “virtuoso conductor”, controlling the sound of a large orchestra, where the orchestra is our whole organism.
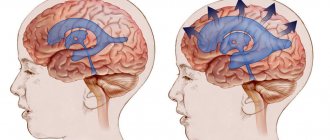
Schematic representation of the location of the tumor.
But, unfortunately, this unique organ, without which a harmonious functional balance in the body is impossible, is not protected from pathological formations or diseases due to hormonal and/or neurogenic disorders. One of the serious diseases is adenoma, in which the glandular, hormonally active epithelium of the pituitary gland of the brain grows pathologically, which can cause disability for the patient.
Anatomy.
Adenomas can be active (AAG) or inactive (NAG). In the first case, the hormonal balance suffers from an excess of secreted pituitary hormones. In the second, the tumor mass irritates, compresses nearby tissues, and the optic nerve is often affected. It is worth noting that greatly increased proportions of the active pathological focus also negatively affect the intracranial tissues located nearby. We suggest you learn about other features of the pathology, including the specifics of treatment, from the article.
Epidemiology: causes, incidence rates
The factor that stimulates the development of a pituitary tumor has not yet been identified, and therefore remains the main subject of research. Experts only voice versions of the probable causes:
- traumatic brain injuries;
- neuroinfection of the brain;
- bad habits;
- pregnancy 3 or more times;
- heredity;
- taking hormonal medications (for example, contraceptives);
- chronic stress;
- arterial hypertension, etc.
The neoplasm is not so rare; in the general structure of brain tumors it accounts for 12.3%-20% of cases. In terms of frequency of occurrence, it ranks 3rd among neuroectodermal neoplasias, second only to glial tumors and meningiomas. The disease is usually benign in nature. However, medical statistics record data on isolated cases of malignant transformation of adenoma with the formation of secondary foci (metastases) in the brain.
The pathological process is more often diagnosed in women (about 2 times more) than in men. Below we present data on the age distribution based on 100% of patients with a clinically confirmed diagnosis. The epidemiological peak occurs at the age of 35-40 years (up to 40%), at 30-35 years the disease is detected in 25% of patients, at 40-50 years - in 25%, 18-35 and over 50 years - 5% each age category.
According to statistics, about 40% of patients have an inactive tumor, which does not secrete excess hormonal substances and does not affect endocrine balance. In approximately 60% of patients, an active formation is identified, characterized by hypersecretion of hormones. About 30% of people become disabled due to the consequences of an aggressive pituitary adenoma.
MRI for Rathke's pouch cyst
The MR picture can be very different (see images below). Although no specific MRI features have been identified, most can be divided into the following two groups:
1) With low signal intensity on T1-weighted images and high signal intensity on T2-weighted images
2) With high signal intensity on T1-weighted images and variable signal intensity on T2-weighted images
T1-weighted sagittal image obtained before contrast enhancement shows a well-defined cyst in the area of the sella turcica, isointense to the cerebrospinal fluid. There is a normal high signal intensity of the posterior lobe of the pituitary gland from behind.
On a T1-weighted coronal image, it is visible just under a centimeter in size at the central part of the sella turcica. Slightly hyperintense compared to cerebrospinal fluid.
On this T2-weighted image, it is isointense to the cerebrospinal fluid.
The large one is hyperintense compared to the cerebrospinal fluid on axial proton-weighted image. There is an expansion of the sella turcica with a lateral deviation of the slightly obscured but patent cavernous part of the internal carotid artery.
Get an MRI of the pituitary gland in St. Petersburg
The contents of the first group resemble cerebrospinal fluid (CSF). In the second group, increased signal on T1-weighted images is associated with high levels of mucopolysaccharides, which is believed to be a consequence of an increase in the number of mucin-secreting cells in the wall, as well as increased activity of these cells.
Rare cases of high signal intensity on T1-weighted images and low intensity on T2-weighted images are associated with a combination of various factors, including the presence of mucopolysaccharides, old hemorrhage, significant cholesterol or cellular debris from the cyst wall. They are almost always homogeneous in signal intensity, while other pathological formations, such as craniopharyngiomas and hemorrhagic adenomas, are more often characterized by heterogeneity in signal intensity.
They have a thin wall that can be contrasted when injecting gadolinium-based contrast agents. Various patterns of gadolinium enhancement may reflect squamous metaplasia of the wall or pituitary tissue displaced to the periphery in the form of a rim.
Data on the relationship between signal intensity and its clinical manifestations are very rare. In 1997, Takeichi and colleagues studied 78 patients, including 9 of their own patients, and reported that cerebrospinal fluid signal intensity was slow growing, large in size, and often caused visual symptoms. Symptomatic ones with different signal intensities were smaller in size.
The DWI-SSFSE mode with ICD values can also be used in the differential diagnosis of craniopharyngiomas and hemorrhagic pituitary adenomas.
Classification of pituitary adenomas of the brain
The pituitary focus is formed in the anterior lobe of the gland (adenohypophysis), which makes up the bulk of the organ (70%). The disease develops when one cell mutates; as a result, it escapes immune surveillance and falls out of the physiological rhythm. Subsequently, through repeated division of the progenitor cell, an abnormal proliferation is formed, consisting of a group of identical (monoclonal) cells. This is an adenoma; this is the most common development mechanism. However, in rare cases, the lesion may initially originate from one cell clone, and after relapse, from another.
Pathological formations are distinguished by activity, size, histology, nature of distribution, type of secreted hormones. We have already found out what type of activity adenomas have - hormonally active and hormonally inactive. The growth of defective tissue is characterized by an aggressiveness parameter: a tumor can be non-aggressive (small and not prone to growth) and aggressive when it reaches large sizes and invades neighboring structures (arteries, veins, nerve branches, etc.).
Large adenoma after removal.
Based on the size of pituitary adenomas, there are the following types:
- microadenomas (less than 1 cm in diameter);
- mesoadenomas (1-3 cm);
- large (3-6 cm);
- giant adenomas (more than 6 cm in size).
According to their distribution, AGGM are divided into:
- endosellar (within the pituitary fossa);
- endoextrasellar (extending beyond the sella landmarks), which extend:
► suprasellar – into the cranial cavity;
► laterosellar – into the cavernous sinus or under the dura mater;
► infrasellar – grow down towards the sphenoid sinus/nasopharynx;
► antesellar – affect the ethmoidal labyrinth and/or orbit;
► retrosellar – into the posterior cranial fossa and/or under Blumenbach’s clivus.
According to histological characteristics, adenomas are given names:
- chromophobe - neoplasia formed by pale, poorly contoured adenohypophyseal chromophobe cells (a common type, represented by NAG);
- acidophilic (eosinophilic) – tumors created by alpha cells with a well-developed synthetic apparatus;
- basophilic (mucoid) – neoplastic formations developing from basophilic (beta cells) adenocytes (the rarest tumor).
Among hormonally active adenomas there are:
- prolactinomas – actively secrete prolactin (the most common type);
- somatotropinomas – produce growth hormone in excess; corticotropinomas – stimulate the production of adrenocorticotropin;
- gonadotropinomas – enhance the synthesis of human chorionic gonadotropin;
- thyrotropinomas – produce a large release of TSH, or thyroid-stimulating hormone;
- combined (polyhormonal) – secrete 2 or more hormones.
Why does a Rathke's pouch cyst form?
As established by Voelker et al., according to the most generally accepted theory of origin, these formations develop from the true residual structures of Rathke's pouch that existed in the prenatal period.
Rathke's pouch appears approximately on the 24th day of embryonic development in the form of a dorsal diverticulum of the oral fossa; this formation is lined with epithelial cells of ectodermal origin. Around the same time, the pituitary funnel is formed in the form of an outgrowth of the neuroepithelium from the diencephalon. By the fifth week it grows to the funnel and becomes obliterated at the buccal-pharyngeal membrane. During the sixth week it separates from the oral epithelium. Then the distal part of the pituitary gland develops from its anterior wall. The posterior wall does not proliferate and remains in the form of a poorly visible intermediate part of the pituitary gland.
The residual lumen of the pocket is reduced to a narrow gap, which, in most cases, disappears. It is believed that persistence and widening of this fissure leads to the development of a Rathke cyst.
Diagram of the development of a cyst from Rathke's embryonic pouch.
Some authors adhere to other theories regarding the formation of these cysts: in particular, according to some theories, the source of development is the neuroepithelium or endoderm, or their development is assumed to be through metaplasia of the cells of the anterior pituitary gland.
Clinical manifestations of the tumor
Many symptoms, as they themselves emphasize, are not taken seriously by patients at first. Ailments are often associated with banal overwork or, for example, stress. Indeed, manifestations can be nonspecific and veiled for a long time - 2-3 years or more. Note that the nature and intensity of symptoms depend on the degree of aggression, type, location, volume and many other characteristics of the adenoma. The neoplasm clinic consists of 3 symptomatic groups.
- Neurological signs:
- headache (most patients experience it);
- impaired innervation of the eye muscles, which causes oculomotor disorders;
- pain along the branches of the trigeminal nerve;
- symptoms of hypothalmic syndrome (VSD reactions, mental imbalance, memory problems, fixation amnesia, insomnia, impaired volitional activity, etc.);
- manifestations of occlusive-hydrocephalic syndrome as a result of blockade of the outflow of cerebrospinal fluid at the level of the interventricular foramen (impaired consciousness, sleep, headache attacks when moving the head, etc.).
- Ophthalmological symptoms of the neural type:
- noticeable discrepancy in visual acuity of one eye from the other;
- gradual loss of vision;
- disappearance of the upper fields of perception in both eyes;
- loss of the visual field of the nasal or temporal areas;
- atrophic changes in the fundus (determined by an ophthalmologist).
- Endocrine manifestations depending on hormone production:
- hyperprolactinemia - discharge of colostrum from the breast, amenorrhea, oligomenorrhea, infertility, polycystic ovary syndrome, endometriosis, decreased libido, hair growth, spontaneous abortions, problems with potency in men, gynecomastia, low quality sperm for conception, etc.;
- hypersomatotropism - an increase in the size of the distal limbs, brow ridges, nose, lower jaw, cheekbones or internal organs, hoarseness and deepening of the voice, muscle dystrophy, trophic changes in the joints, myalgia, gigantism, obesity, etc.;
- Itsenko-Cushing syndrome (hypercortisolism) - dysplastic obesity, dermatoses, osteoporosis of bones, fractures of the spine and ribs, dysfunction of the reproductive organs, hypertension, pyelonephritis, stretch marks, immunodeficiency states, encephalopathy;
- symptoms of hyperthyroidism - increased irritability, restless sleep, changeable mood and anxiety, weight loss, trembling hands, hyperhidrosis, irregular heart rhythm, high appetite, intestinal disorders.
Approximately 50% of people develop symptomatic (secondary) diabetes as a result of a pituitary adenoma. 56% are diagnosed with loss of visual function. To one degree or another, almost everyone experiences symptoms classic for pituitary hyperplasia of the brain: headache (more than 80%), psycho-emotional, metabolic, cardiovascular disorders.
Forecast and possible consequences
Adverse consequences of surgical treatment of a brain cyst formed on the pituitary gland are relatively rare. Typically, postoperative disorders are associated with the development of diabetes mellitus (4% of cases), secondary infection (4% of cases), rhinorrhea - copious discharge of mucous substrate from the nasal passages (7% of cases).
The prognosis is relatively favorable - in most cases (about 80%), the operation leads to relief from the manifestations of the pathology. If a large cyst is not treated, complications may develop - loss of vision, seizures, hormonal imbalance, increased intracranial pressure.
A pituitary cyst is a space-occupying formation, which is often a congenital pathology of brain development. Less commonly, a cystic cavity is formed as a result of previous infections, injuries in the head area, or neurosurgical interventions. Most often it is asymptomatic. If severe neurological symptoms occur, surgical removal is usually performed.
154
Methods for diagnosing pathology
Specialists adhere to a unified diagnostic scheme if a person suspects this diagnosis, which includes:
- examination by a neurologist, endocrinologist, ophthalmologist, ENT doctor;
- carrying out laboratory tests - general blood and urine tests, blood biochemistry, blood tests for sugar and hormone concentrations (prolactin, IGF-1, corticotropin, TSH-T3-T4, hydrocortisone, female/male sex hormones);
- heart examination using an ECG machine, ultrasound of internal organs;
- ultrasound examination of the vessels of the veins of the lower extremities;
- X-ray of the skull bones (craniography);
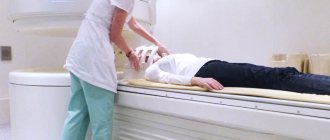
- computed tomography of the brain, in some cases there is an additional need for MRI.
MRI.
Note that the specificity of collecting and studying biological material for hormones is that conclusions are not drawn after the first examination. To ensure the reliability of the hormonal picture, dynamic observation is necessary, that is, you will need to donate blood for research repeatedly at certain intervals.
Brief description of symptoms
For a long time, hormonally inactive formations do not attract attention. As long as their size does not put pressure on neighboring areas, there are no symptoms of the disease. As the pituitary cyst grows, the surrounding tissues experience uncompensated effects. This determines a number of characteristic symptoms:
- deterioration of vision, up to the development of blindness;
- intense headache;
- increased fatigue;
- weight loss;
- nervousness, vulnerability, tendency to emotional breakdowns.
Deterioration in vision is due to the fact that the optic nerve is anatomically located next to the pituitary gland. Therefore, when it is compressed, lateral vision is first limited, then general vision, then complete blindness occurs. The second form - hormonally active cysts - depends on increased hormone synthesis.
Fertility suffers from an excess of prolactin; adrenocorticotropic hormone leads to hypertensive symptoms, osteoporosis, increased hair growth on the body and face, the development of diabetes and other dangerous diseases.
If there is a lot of growth hormone, there is a risk of developing acromegaly. Characteristic signs: an increase in the length of the feet, hands, disproportionate size of the brow ridges, ears and other defects. Hypersynthesis of thyrotropin is a signal for arrhythmia, bulging eyes, and hand tremors. In this case, the primary symptoms are severe weight loss, arrhythmia, and a sharp loss of strength. With hyperfunction of hormones, diabetes insipidus can develop.
Hormonally active cysts cause the following complaints:
- dry skin;
- drowsiness;
- increased fatigue;
- hyperfunction of the kidneys to produce urine and corresponding thirst;
- hypotension;
- decreased vital activity;
- sexual disorders.
With all the variety of symptoms, the clinic may differ in each specific case. This is determined by the individual characteristics of the body and the degree of neglect of the process.
It happens that a person has had a cyst since childhood, but it did not bother him until the onset of age-related diseases: hypertension, cerebral atherosclerosis, cardiac and pulmonary pathologies.
Principles of disease treatment
Let’s make a reservation right away: with this diagnosis, the patient needs highly qualified medical care and constant monitoring. Therefore, there is no need to rely on chance, believing that the tumor will resolve and everything will pass. The outbreak cannot go away on its own! In the absence of adequate therapy, the danger of becoming disabled with irreversible functional impairment is too great, and deaths from the consequences also occur.
Depending on the severity of the clinical picture, patients are recommended to solve the problem surgically and/or conservative methods. Basic therapy procedures include:
- neurosurgery
- removal of adenoma by transnasal access (through the nose) under endoscopic control or transcranial method (standard craniotomy is performed in the frontal part) under the control of a fluoroscope and microscope;
90% of patients are operated on transnasally, 10% require transcranial ectomy. The latter tactic is used for massive tumors (more than 3 cm), asymmetric growth of newly formed tissue, extension of the lesion beyond the sella, and tumors with secondary nodes.
- treatment with medications
- the use of drugs from a number of dopamine receptor agonists, peptide-containing agents, targeted drugs for hormone correction; - radiotherapy (radiation treatment)
– proton therapy, remote gamma therapy using the Gamma Knife system; - Combination treatment
– the program course combines several of these therapeutic tactics.
The doctor may not use surgery, but recommend observation of a person diagnosed with pituitary adenoma in the absence of focal neurological and ophthalmological disorders and the tumor is hormonally inactive. Such a patient is managed by a neurosurgeon in close collaboration with an endocrinologist and an ophthalmologist. The patient is systematically examined (1-2 times a year), sent for MRI/CT, eye and neurological examinations, and measurement of hormones in the blood. In parallel with this, the person undergoes courses of targeted maintenance therapy.
Since surgery is the leading method of treating pituitary adenoma, we will briefly highlight the course of the surgical process of endoscopic surgery.
Definition
It is a round formation of regular shape, located in the area of the foramina of Monroe, causing the development of hydrocephalus with signs of increased intracranial pressure.
Macroscopically, the cyst looks like a thin-walled formation located in the area of the fornix or foramina of Monroe, attached to the choroid plexus or nearby brain structures. The size of the cysts ranges from 0.3 to 3-4 cm. The contents of the cysts are viscous, yellow-green in color. Microscopically, their inner wall is represented by one layer of cylindrical ciliated epithelium. Its cells produce mucin. Colloid cysts are characterized by a long course and a slow increase in their volume. With total removal, no relapses are observed, cerebrospinal fluid circulation is normalized and hydrocephalus is reduced.
Transnasal surgery to remove pituitary adenoma of the brain
This is a minimally invasive procedure that does not require a craniotomy and does not leave behind any cosmetic defects. It is most often performed under local anesthesia; the surgeon’s main instrument will be an endoscope. A neurosurgeon removes a brain tumor through the nose using an optical device. How is all this done?
- The patient is in a sitting or semi-sitting position during the procedure. A thin endoscope tube (no more than 4 mm in diameter), equipped with a video camera at the end, is carefully inserted into the nasal cavity.
- The real-time image of the lesion and adjacent structures will be transmitted to the intraoperative monitor. As the endoscopic probe advances, the surgeon performs a series of sequential manipulations to get to the part of the brain of interest.
- First, the nasal mucosa is separated to expose and open the anterior wall. The thin bony septum is then cut. Behind it is the desired element - the sella turcica. A small hole is made in the bottom of the sella turcica by separating a small fragment of the bone.
- Next, using microsurgical instruments placed in the channel of the endoscope tube, pathological tissues are gradually chipped off through the access created by the surgeon until the tumor is completely eliminated.
- At the final stage, the hole created in the bottom of the saddle is covered with a bone fragment, which is fixed with special glue. The nasal passages are thoroughly treated with antiseptics, but not tamponed.
The patient is activated in the early period – already on the first day after low-traumatic neurosurgery. About 3-4 days later, you will be discharged from the hospital; then you will need to undergo a special rehabilitation course (antibiotic therapy, physiotherapy, etc.). Despite undergoing surgery to excise a pituitary adenoma, some patients will be asked to additionally adhere to hormone replacement therapy.
The risks of intra- and postoperative complications during the endoscopic procedure are reduced to a minimum – 1%-2%. For comparison, negative reactions of various types after transcranial resection of AGGM occur in approximately 6-10 people. out of 100 operated patients.
After a transnasal session, most people experience difficulty in nasal breathing and discomfort in the nasopharynx for some time. The reason is the necessary intraoperative destruction of individual structures of the nose, resulting in painful symptoms. Discomfort in the nasopharyngeal area is usually not regarded as a complication if it does not intensify and does not last long (up to 1-1.5 months).
The final assessment of the effect of the operation is possible only after 6 months using MRI images and the results of hormonal tests. In general, with timely and correct diagnosis and surgical intervention, high-quality rehabilitation, the prognosis is favorable.
CT scan for Rathke cyst
On CT, the cyst usually appears as a well-circumscribed hypodense sella turcica, sometimes extending into the suprasellar space. Due to their different contents, they can be isodense or hyperdense relative to the brain parenchyma.
They are usually characterized by a thin wall that can accumulate contrast. Significant differences in the contrast enhancement of different cysts on CT images may reflect squamous metaplasia of the wall or peripheral displacement of pituitary tissue in the form of a rim. Extravasation of cyst contents can cause inflammation of the underlying structures, which leads to the accumulation of contrast.
Calcifications are rare. Complex cysts may have internal septations. Large ones can cause restructuring of bone tissue, expansion of the sella turcica, and osteoporosis of its back.
CT images are not enough to confirm the diagnosis, since the data obtained do not allow us to clearly exclude other pathology. Simple cysts may be indistinguishable from arachnoid or epidermoid cysts. More complex ones can be difficult to distinguish from craniopharyngioma or pituitary adenoma. Therefore, the diagnosis must be made based on a combination of clinical, biochemical, pathological and radiological data.
What is the danger
With advanced and aggressive progression of a benign neoplasm that is not subject to therapeutic measures, life-threatening and irreversible consequences can develop.
Due to the fact that the tumor adversely affects hormonal levels, hormone-dependent pathologies will begin to develop first. In the case of representatives of the fair sex, a cyst in the pituitary gland often provoked the formation of benign cavities in the chest and other diseases affecting the reproductive organs.
In addition to the described complications, cystoma causes the formation of the following diseases:
- Hyperprolactemia;
- Acromegaly;
- Itsenko-Cushing's disease;
- Thyrotoxicosis.
In order to prevent negative diseases, medical experts recommend carefully monitoring your well-being and undergoing a full preventive examination every year.