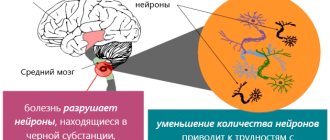
Parkinson's disease is a progressive disease of the nervous system in which neurons in the brain that regulate motor function are destroyed. The exact cause of the pathology is still unknown, but doctors suggest that hereditary predisposition, age-related changes and the influence of chemicals play a central role.
The main symptoms are tremor (shaking) of the hands and head, impaired speech and facial expressions, changes in gait, stiffness of movements, and in the final stages dementia appears. A characteristic feature of progressive Parkinson's disease is the development of depression in older people.
general information
Parkinson's disease affects men twice as often as women. In most cases, it develops after 60 years of age, but cases of early onset of the disease are recorded (at 30-40 years of age), as well as juvenile forms that develop in people in their twenties.
There are several hypotheses for the formation of pathology. It has now been precisely proven that one of the mechanisms of its development is the gradual degeneration of neurons and a decrease in the production of dopamine. It is an important neurotransmitter involved in the transmission of nerve impulses. As a result, a specific set of disorders is formed, which makes it easy to make a diagnosis.
results
Qian and colleagues found that depletion of PTB1 in astrocytes stimulates their transformation into neurons. The outcome of this transformation depends on the region of the brain in which the target astrocytes are located. Astrocytes in the midbrain were found to synthesize low levels of the transcription factors Lmx1a and Foxa2. These factors are markers of dopaminergic neuron precursors during midbrain development. However, depletion of PTB1 protein increased the production of these transcription factors in midbrain astrocytes. On the other hand, exposure to cortical astrocytes led to an increase in the synthesis of transcription factors characteristic of cortical neurons.
After 4 weeks of exposure to astrocytes in vitro, 50–80% of the cells became morphologically neuron-like and tested positive for the neuronal markers TUJ1 and MAP2.
In in vivo experiments, 3 weeks after shPTB administration, about 20% of cells that received red fluorescent protein synthesized the neuronal marker NeuN. After 10 weeks, there were already about 80% of cells with NeuN. At the same time, the cells stopped producing GFAP, a protein present in astrocytes.
After 12 weeks, 30–35% of these cells had transformed into dopaminergic neurons. In other words, reprogramming astrocytes helped restore more than 600 dead dopaminergic neurons (Figure 4).
Figure 4. Brain of a mouse with “unilateral” Parkinson's disease. Above - before reprogramming using shPTB. Below - after. Dopaminergic neurons are shown in green.
One-Time Treatment Generates New Neurons, Eliminates Parkinson's Disease in Mice
Moreover, the newly created neurons began to actively produce dopamine. Exposure to 6-OHDA reduced neurotransmitter levels to 25% of normal levels. After therapy, dopamine concentrations rose to 65% of normal levels.
Causes
The exact causes of Parkinson's disease have not been identified. Scientists have been able to identify only a number of factors that increase the risk of developing neurodegenerative processes:
- natural processes of aging of the body, accompanied by a decrease in the ability of tissues to regenerate;
- genetic predisposition (this is especially true for forms with early onset);
- chronic lack of vitamin D, which protects neurons from the pathological effects of toxins;
- intoxication with salts of heavy metals, pesticides, alcohol;
- poor environmental conditions in the region of residence;
- taking certain medications (for example, chlorpromazine);
- chronic insufficiency of blood supply to the brain (due to atherosclerosis, osteochondrosis of the cervical spine, etc.);
- infections affecting the central and peripheral nervous system (meningitis, encephalitis, herpes viruses, influenza);
- brain tumors;
- traumatic brain injuries;
- smoking;
- frequent and prolonged stress, chronic fatigue.
Make an appointment
Astrocytes - a possible cure?
Recently, several scientific papers have been published on the transformation of various cells into dopaminergic neurons. Basically, the researchers chose astrocytes - cells that support the vital activity of neurons. They got their name from their characteristic star-shaped shape (Fig. 2). The responsibilities of these cells include providing the metabolic needs of neurons, participating in the timely release of neurotransmitters from nerve endings, storing nutrients and regulating neuronal activity.
Figure 2. Auxiliary cells of the nervous system - astrocytes. Normally, they ensure the vital activity of neurons, but by influencing the molecular processes occurring in astrocytes, they can be “transformed” into neurons of any type.
Human Astrocytes
But most of all scientists are interested in their reparative function. In 2014, a group of scientists from Lund University and Karolinska Institutet discovered that when nerve tissue is damaged after a stroke, astrocytes are able to replace dead neurons [12]. At the same time, the Notch1 signaling pathway, which is of key importance in the processes of cell proliferation and differentiation, “turns off” in them. In a healthy brain, this pathway is active and blocks the conversion of astrocytes into neurons. However, after a stroke, this mechanism is suppressed, and astrocytes can begin to transform into neurons.
The idea for the study, conducted by a team from the University of California, is not new. Back in 2021, a group of Swedish scientists turned human astrocytes into neurons in vitro using viral vectors. After this, mouse astrocytes were also transformed, but in vivo. For reprogramming, they used three transcription factors (NEUROD1, ASCL1 and LMX1A) and miR218 microRNA [13]. Astrocyte transformation in vitro has been improved using molecules capable of inducing chromatin rearrangement and activating several signaling pathways.
However, a 2021 study proposes a much simpler method for reprogramming astrocytes: simply blocking the production of a single protein.
Symptoms
Parkinson's disease is manifested by specific symptoms, which together form a clear picture of the disease:
- tremor: fine trembling begins in one hand and then spreads to both limbs and head; the movement of the fingers is somewhat reminiscent of counting coins; while performing purposeful movements (for example, while working at a computer);
- general slowness of movements (bradykinesia): the patient often freezes in one position;
- specific gait: a person moves with small, shuffling steps, as if he is constantly on very slippery ice;
- poor facial expressions: a mask effect is formed due to low mobility of facial muscles;
- monotonous, quiet speech;
- increased muscle tone (muscle rigidity): the figure becomes stooped, arms and legs are slightly bent, the head is tilted forward;
- postural instability: a person has difficulty starting and ending movements, resulting in decreased ability to maintain balance;
- disorders of the autonomic nervous system: oily skin, excessive secretion of saliva and sweat;
- decreased sense of smell;
- constipation, urinary disorders.
Unlike other neurodegenerative diseases, Parkinson's disease has little effect on intelligence in early and middle stages of development. As the pathology progresses, there is a decrease in the speed of thinking and speaking, decreased mood, depression and indifference to everything that happens.
How the disease develops
There are five main stages in the development of Parkinson's disease.
- First. The disease is expressed by weak tremors of the limbs. This condition does not prevent a person from leading a full, normal lifestyle, but in some cases he may experience discomfort.
- Second. Trembling of hands and feet increases. In the second stage, the patient is still able to lead a normal life, but with minor restrictions.
- Third. Against the background of progressive symptoms, severe tremor is noted, and the patient experiences loss of coordination in space. But even in this state, a person does not lose the skill of self-care, despite the fact that his movements slow down significantly and he quickly gets tired.
- Fourth. Serious motor and speech disturbances are noted. The patient can only partially take care of himself, so he needs help from outsiders. At the fourth stage of the disease, damage to the facial muscles occurs, which leads to loss of facial expressions. Also at this stage, problems with the bladder may appear.
- The sick person experiences complete loss of movement. The fifth stage is characterized by the rapid development of such deviations as memory impairment. The patient may experience hallucinations. A patient in this condition requires constant care. It is best for such a patient to be hospitalized.
Parkinson's disease is a progressive disease, so the transition from one form to another occurs quite quickly. In each individual case, it depends on the individual characteristics of the patient’s body. The degrees of development of the disease also vary:
- the disease remains at one stage for a long time: sometimes there is no progression for more than 10 years;
- a moderate pace of development is characterized by a transition to a new stage within 5 years;
- rapid transition to a more severe form, which occurs over 1.5-2 years.
Thanks to modern techniques, treatment and care for Parkinson's disease helps to significantly slow down the progression of the disease. Experienced doctors are able to identify possible deviations, which also makes therapy more effective.
Stages of development
Currently, doctors distinguish 5 stages of Parkinson's disease, manifested by a certain set of symptoms:
- Stage 0: no clinical manifestations;
- Stage 1: there are slight difficulties in moving one arm, a slight tremor, first with excitement, then at rest; the sense of smell and sleep are disrupted, fatigue and apathy appear;
- Stage 2: disturbances involve the second hand, trembling of the tongue and lower jaw appears; salivation; hypokinesia is formed; the ability to self-care is preserved;
- Stage 3: stiffness and impoverishment of movements increases, facial expressions are almost absent; a specific gait and posture is formed; during a conversation, the patient begins to get stuck on the same word; self-care is difficult, but possible;
- Stage 4: postural instability develops, the patient begins to fall frequently; the intellect begins to suffer, depression increases; At this time, suicide attempts are common; a person needs help to perform simple actions;
- Stage 5: manifestations reach their climax, the person cannot sit down, stand up and walk independently, eating is difficult due to swallowing disorders; loss of control over bowel movements and urination; the patient requires constant care.
Diagnostics
The diagnosis of Parkinson's disease is made based on the characteristic clinical picture. The patient is examined by a neurologist who notes typical signs. Complaints must be clarified and an anamnesis of the disease (history of the appearance of symptoms) and life history (information about previous injuries, chronic diseases, surgical interventions) is collected. Laboratory and instrumental diagnostics are used to clarify concomitant pathology and exclude other causes of neurological disorders.
Treatment of Parkinson's disease
At the moment, successfully selected treatment for Parkinson's disease can stop the process of degradation and reduce the severity of pathological symptoms. The disease begins to progress more slowly, which allows patients to stay in good shape longer.
Drug treatment
Drug treatment is aimed at restoring the balance of dopamine in the central nervous system. The following drugs are used:
- Levodopa and its analogues: serve as the basis for the production of dopamine;
- dopamine receptor agonists: stimulate receptors similar to a natural neurotransmitter and reduce the severity of symptoms;
- MAO-B inhibitors: reduce the breakdown of dopamine;
- COMT inhibitors: prescribed in combination with levodopa and reduce its breakdown;
- anticholinergics: aimed at reducing symptoms.
There are combination products that combine several active ingredients for the fastest possible effect.
Non-drug treatment
Drug treatment is complemented by physiotherapy, exercise therapy and massage. Physiotherapy is used to activate metabolic processes and increase blood flow in the brain. Depending on the patient’s condition and concomitant diseases, the following may be prescribed:
- magnetic therapy;
- ultrasound stimulation;
- electrosleep;
- mineral baths;
- acupuncture.
The massage is aimed at improving motor activity. Intensive muscle kneading and passive gymnastics reduce muscle stiffness and have a general strengthening effect.
Physical therapy exercises allow you to:
- reduce muscle stiffness and strengthen it;
- increase sense of balance;
- improve the patient's emotional state.
Most exercises are aimed at training the sense of balance. The complex is selected individually depending on the patient’s condition, age and concomitant diseases.
Surgery
The help of surgeons is relevant in the last stages of the disease. The most effective and safest operation is the installation of a brain stimulator. The intervention does not require opening the skull. Thin electrodes are inserted into the brain and a small stimulator is placed under the skin of the collarbone. The device is programmed for a specific pulse frequency; in addition, the patient and his relatives can change the settings depending on the condition. Using a stimulant allows you to reduce the dosage of medications and keep symptoms under control for a long time.
Other surgical treatment options require working on an open brain:
- thalamotomy: destruction of part of the thalamus, allowing to get rid of tremors, but maintaining other symptoms;
- pallidotomy: partial removal of one area of the brain (globus pallidus), significantly reducing all the main symptoms of the pathology.
One of the most significant motor disorders in patients with Parkinson's disease (PD) is walking impairment. It is its severity that largely determines the severity of the condition and the patient’s quality of life. As a result of numerous domestic and foreign studies [1-5], it has been established that walking impairment is an independent manifestation of PD, which has a special pathogenesis, requiring a specific approach to correction, and which can probably be considered the fifth cardinal sign of parkinsonism along with hypokinesia, rigidity, and tremor rest and postural disorders.
Gait disturbances in patients with PD at the onset of the disease depend on hypokinesia and rigidity. As a result, there is a slow initiation of walking, a decrease in its speed and a decrease in the size of the step (microbasis). The gait becomes shuffling, while the patient almost does not lift his feet off the floor, and a “mincing” step occurs. Poor posture in PD occurs as a result of constant flexion of the cervical spine and the formation of a “bent” position of the torso with kyphosis. When walking, the torso may lean further forward. To maintain balance and avoid falling, patients, trying to “catch up” with the body’s center of gravity, are forced to gradually accelerate (propulsion). Some patients experience retro- and lateropulsions. Quite often in PD, the phenomenon of freezing when walking occurs .
.: freezing). As PD progresses, freezing may occur more frequently and last longer, significantly impairing walking.
The role of postural instability is especially significant at the late stage of the disease, when a shift in the patient’s center of gravity does not cause compensatory movements of the trunk and limbs, which leads to falls [3, 6, 7].
There are three types of hypokinesia in parkinsonism [8]. The first type is secondary slowness (secondary akinesia), caused by muscle rigidity and manifested by a violation of reciprocal and rapid repetitive movements (acheirokinesis, difficulties in alternating pronation-supination). In the second type, there is an impoverishment of fine and automated movements, which does not depend on the severity of muscle rigidity (primary akinesia). These two types of hypokinesia can be treated with levodopa. The third type is characterized by a lack of motivation to act and is manifested by difficulties in starting movement (initiation of walking). Treatment with levodopa drugs not only does not reduce, but can even lead to an increase in the severity of this phenomenon, which largely depends on the dysfunction of nondopaminergic mechanisms [5, 9, 10].
A decrease in general activity in combination with a kyphotic alignment of the spine and general hypokinesia, as a rule, leads to respiratory disorders.
Problems in diagnosing gait disorders in PD.
The mechanisms of development of gait disturbances in various diseases and the possibility of their compensation, as well as the dynamics of changes in gait disturbances and assessment of the results of therapy suggest the development of instrumental methods of gait analysis [11]. Various ichnometric techniques are used to record the spatial characteristics of a step. Currently, the most common is the rather labor-intensive impregnation method of ichnometry, which consists of manually measuring the distance between foot prints on a paper strip [12, 13]. Mechanical methods of ichnometry using ink recorders attached to shoes are also inconvenient. Optoelectronic methods are difficult to use and expensive. Electrical ichnography methods are more accessible, but most of them require the use of special shoes or the connection of electrodes attached to the patient’s shoes and a reading device using a cable, which changes the walking pattern and is reflected in the final results of the study [14-17]. In this regard, the tasks of creating a simple but sufficiently informative method for recording gait and selecting criteria for assessing gait in order to more fully understand its mechanisms in health and pathology are extremely relevant.
To objectify walking parameters, the authors, together with the experimental design bureau of the Zheleznogorsk Mining and Chemical Combine, Krasnoyarsk Territory, created a device for determining a person’s walking-speed characteristics (USHCH)1. This is a 10 m long contact track with electrical sensors that respond to closure with a special self-adhesive contact disk made of aluminum foil with a diameter of 5 mm. One contact sensor is attached to symmetrical points of the subject’s shoes. When walking, the contacts are closed, the corresponding information enters the computer data conversion unit, which allows you to analyze the length of each step, the time spent on a step, the frequency distribution of various step lengths, the speed of a person’s movement, as well as save and statistically process the data obtained. The advantages of the USHCH include the use of familiar shoes by the subject and the absence of influence on the gait of the contact sensor, as well as the speed and convenience of saving data in electronic form.
Typical graphs of the results of examination of healthy young, healthy elderly patients with BE are presented in Fig. 1. From the presented graphs it is clear that healthy young people are characterized by a significant standardness of gait (accordingly, low variability in step length) with small fluctuations relative to the average. As a result, the graph has the shape of a pyramid with a narrow base. In healthy elderly people, the base of this pyramid expands due to a greater spread in step lengths (greater variability in step length). In a patient with PD, the term “mincing gait” is objectified, with marking time and phenomena of motor fluctuations in the form of freezing, which is manifested by a large number of steps of minimal length.
Rice. 1. Typical graphs of survey results. a - healthy people aged 19-29 years; b - healthy aged 56-75 years; c — patients with B.P. Here and in Fig. 2: the x-axis is the step length (cm), the y-axis is the frequency.
Another diagnostic assessment approach is to formalize and standardize the examination without the use of special devices. For example, the time it takes to travel a certain distance is studied or, conversely, the distance traveled per unit of time is determined.
Methods of tempo-rhythm gait correction (TRCG)
Already since the 19th century, the phenomenon of paradoxical kinesia has been known, which consists of a decrease in oligobradykinesia under the influence of periodization of time (the sound of a march, rhythmic commands, etc.) or space (steps, floor with contrasting floorboards, etc.) [9, 18, 19]. Attempts have been made to improve the patient's walking using these methods. Thus, the positive effect of patients walking to music was described, consisting in an increase in step length [20, 21]. The technique of dynamic visual afferentation [7] has also shown significant effectiveness, but its non-laboratory use is currently difficult. Meta-analyses have shown that sound stimulation is more effective than visual stimulation in terms of restoring walking, since it affects not only step length (like visual stimulation), but also its speed and rhythm [22-25]. In addition, audio stimulation is more accessible for subsequent use in order to maintain the positive effect [25, 26].
M. Thaut et al. [21] proposed using a single frequency of sound signals (the sound of a metronome recorded over music in the basic mode and accelerated by 10%) to correct gait disturbances in patients with PD, which did not take into account the individual characteristics of the patients and the degree of gait disturbance. The study involved a total of 21 patients with stages II-IV of the disease according to Hoehn-Yahr. This, obviously, somewhat blurs the results of the study, preventing a full study of the effectiveness of therapy at different stages of the disease. The authors assessed the results of correction during both on and off periods. A significant difference from most other works is the objectification of walking with the placement of markers on four points of each foot using a computerized installation, which significantly increases the reliability of the data. In general, relatively little effectiveness of the treatment method used was demonstrated. The authors also stated that simply changing step length is not enough to significantly improve walking.
In our opinion, special attention in the restorative treatment of walking disorders in parkinsonism should be paid to the patient’s individual abilities to maintain different walking paces, their dynamic adjustment depending on the stage of the disease and drug regimens, based on quantitative registration of step parameters during the treatment process [25 -27].
We have developed and put into practice a method for treating walking disorders in parkinsonism, which consists of using a TRK, based on the principle of RB2. After a detailed study of the walking parameters, the patients were tested to select the individual frequency of external stimulation, which was carried out as follows: the patient was asked to walk under a frequency specified by the sound in the range from 40 to 120 signals per 1 minute (each subsequent passage was carried out with an increase in frequency by 5 signals per 1 minute ), synchronizing your steps with the sound signal. If the patient was not able to synchronize the step with the sound signal when the frequency increased, testing was stopped. The study was carried out at the USHCH with the determination of linear walking parameters. Changes in the motor pattern of walking were visually assessed, and the adequacy of physical activity was monitored by determining the patient's heart rate and blood pressure. The optimal rhythm was considered to be one in which the step length was greatest with fewer steps and the patient feeling comfortable. During the course of treatment, an individually selected optimal rhythm of sound signals was recorded on an audio cassette, which the patient could use while walking using a portable audio player and headphones. To record the optimal frequency (FO) and then use it in classes with patients, we used a flash player and an electronic device we developed for training movements3.
Classes to correct walking with step synchronization under OC were carried out daily for 5-20 minutes, 3 to 6 times a day, depending on the patient’s condition. Thus, during walking synchronized with the tempo of sound stimulation, the motor stereotype of pathological gait changed, which, however, quickly faded during independent walking at an arbitrary pace without reinforcement by exogenous stimulation. In order to consolidate the achieved effect, patients were given an audio cassette with an OC recorded on it, which they used while walking using an audio player and headphones. RFs were also recorded on the patients’ flash player or given a device we developed for walking training, which the patients used in everyday life (walking around the apartment, walking on the street, etc.).
According to our observations, in some patients with PD, the effect of using sound stimulation with OC in relation to a sharp increase in step length was comparable to the effect of levodopa drugs. We present graphs of the results of assessing the walking of a patient with stage III PD at a free pace and synchronized with the frequency of the supplied sound signals (Fig. 2). The results are also presented in table. 1 and 2.
Table 1. Results of a gait study in patients with stage II PD according to Hoehn-Yahr Note. KVS is the coefficient of step variability, ADS is the average step length (cm).
Table 2. Results of a gait study in patients with stage III PD according to Hoehn-Yahr
Rice. 2. Results of examination of patient M., with stage III PD according to Hoehn-Yahr. a — in free tempo mode; b - in walking mode, synchronized with sound signals supplied from the OV.
It was found that the TRKH method, used in the treatment regimens for patients with PD stage II according to Hoehn-Yahr, showed higher efficiency in gait restoration than in the comparison group. The positive dynamics of walking indicators (WDS, KWS) exceeded those in patients with PD with stage II according to Hoehn-Yahr, in whose treatment only antiparkinsonian drugs were used. Walking disorders in patients with PD with stage III according to Hoehn-Yahr turned out to be more pronounced compared to patients with stage II. In this regard, the need to restore walking in these patients was significantly higher. The positive effect of the use of TRKH in patients with stage III PD also turned out to be significantly higher compared with those receiving antiparkinsonian drugs.
Stimulus selection has been carried out in other studies, in particular, an attempt was made to improve the quality of walking in patients with early stages of PD using TRX with an audio signal that was selected individually [28]. To do this, the patient walked a distance of 9 m 15 times at a comfortable speed, the average rhythm value was calculated (the authors do not indicate which objective control methods were used), and then the patient was asked to walk using the given rhythms. The value of the work is reduced by the small number (11 people) of those examined and the impossibility of extrapolating the data obtained to patients with late stages of BP. This is due to the fact that during moments of severe hypokinesia the patient’s rhythm can be very low, and increasing it is unlikely to significantly change walking. In our opinion, the disadvantage of the work is the underestimation of other walking parameters.
An alternative option for TRKH is the use of a special device that sets the rhythm of movement for the patient through weak electrical impulses, alternately (alternating between the left and right legs) irritating the skin and causing weak muscle contraction4. The device is attached to the patient's belt, electrodes are installed on the front surface of both thighs (one for each leg). The strength of irritation is individually set ,
which is determined by subjective sensations: there should be a clear sensation of the signal passing and slight tension in the muscles of the anterior thigh. Irritation should not be accompanied by subjective unpleasant sensations. Then the stimulation rhythm is set in the range from 60 to 110 pulses per 1 minute in increments of 5 pulses per 1 minute (a range different from that for sound stimulation is associated with the technical capabilities of the device). Electrical impulses alternately stimulate the right and left limbs. The patient is asked to synchronize steps with the given stimuli. Testing is stopped if the patient is unable to synchronize when the frequency increases. The study was carried out at the USHCH, the change in the motor pattern of walking was visually assessed, the adequacy of physical activity was monitored by determining the patient's heart rate and blood pressure. The optimal rhythm was considered to be the one with the longest step length with fewer steps and a comfortable state of health.
This stimulation technique was used in a relatively small number of patients with PD, however, in one of the monocenter studies in patients with vascular parkinsonism (VP) with the presence of active (sound stimulation) and passive (no impact) control, a significant increase in SRS and a decrease in EPS were found. The results are presented in more detail in Table. 3.
Table 3. Data on the initial state of walking in subgroups of SP Note. * — differences are significant when compared with the comparison group (p<0.01).
The effectiveness of both techniques, in our opinion, should be associated with the mobilization of additional compensatory mechanisms in the form of cortical control of walking, implemented through the premotor cortex, as well as, possibly, the additional influence of the cerebellar pathways, compensating for the dysfunction of the basal ganglia and the associated additional motor cortex. One of the arguments in favor of this concept is the work of M. Mak et al. [29], who showed a significant deterioration in walking compared to healthy people in patients with PD when their attention was diverted to calculations, and, on the contrary, a greater level of positive changes (length, speed and rhythm of walking) in patients with PD compared to healthy people when presented with audiovisual incentives. Walking was significantly improved by using both auditory and visual stimuli, but their combination did not significantly increase the effect of TRKH [30]. This may be explained by the individual dominance of the patient’s perception of signals of one type or by elements of distraction when using two signals of different types, which did not allow the positive effect to be summed up.
In a single-blind study on a large group of 153 patients, data were obtained on a small (about 4.2%) but significant reduction in episodes of “freezing” walking when using the methods used by A. Nieuwboer et al. [31]. Similar data were obtained from a study of the effectiveness of audio and visual stimulation [32]. A significant effect on freezing gait (10% reduction) and a higher level of evidence were demonstrated in one of the newest studies in this field [10]. It had a randomized crossover design: for 2 weeks, the experimental group was engaged in walking with stimulation of an individually selected modality (audio, visual, tactile - cane, mental - music), and the comparison group did not receive such therapy. Despite the relatively small sample (23 patients were randomized), the results are beyond doubt, given the carefully designed design, strict requirements for the inclusion of patients (51 were screened) and competent statistical processing.
Methods of exercise therapy and occupational therapy in the treatment of PD
The thesis about the benefits of breathing exercises for patients with PD is confirmed by the data from the work of R. Inzelberg et al. [33]. The patients were divided into a comparison group (false breathing exercises) and a main group (breathing exercises). Significantly better results were in the main group.
Considering programs aimed at exercises for skeletal muscles from the perspective of evidence-based medicine, it can be stated that any systematic exercise, including independent home exercise, has a positive effect on motor functions [34]. A meta-analysis conducted by the Cochrane Movement Disorders Group (33 studies, 1518 patients) fully confirms the value of exercise therapy compared to sham (or no intervention) in relation to speed and step length, balance and motor functions [35]. Systematic exercises under the supervision of exercise therapy specialists are significantly more effective than independent exercises at home and have a positive effect on motor functions (including UPDRS scale scores, assessed using the PDQ-39) and daily activity (assessed on the NEADL scale) [36]. The use of a specialized exercise program (ParkFit) in 540 patients with PD with reduced mobility in a randomized controlled trial did not show a significant effect on activity on the LAPAQ scale, but other secondary outcome measures (including an activity diary and a 6-minute walk test) showed significant improvement [37]. We emphasize that the study concerns not just patients with PD, but patients with reduced motor activity. Exercise therapy does not have a significant effect on the incidence of falls [35, 38]. Occupational therapy has a positive effect on quality of life (assessed by PDQ-39), daily activity (assessed by NEADL) [15].
Transcranial magnetic stimulation (TCMS)
After the first optimistic isolated reports on the use of TCMS for patients with PD, studies appeared indicating the low effectiveness of this method [39]. Apparently, the problem is related to the selection of the optimal stimulation mode, primarily the frequency of stimulation and its area: for example, in this study, a frequency of 50 Hz was used. Another randomized trial [40] had a comparison group (sham TMS) and two intervention groups with a frequency of 10 and 1 Hz. The data obtained indicate a significant improvement (about 6.8 points on the UPDRS scale) in the group receiving stimulation with a frequency of 1 Hz, while stimulation with a frequency of 10 Hz had a much smaller and unstable effect. An open-label, uncontrolled study [25] demonstrated the effectiveness of 9 Hz TCMS in combination with pharmacotherapy (galantamine) in patients with PD and dementia. Interestingly, positive dynamics were observed (increased metabolism of the corresponding structures of the nervous system) according to positron emission tomography.
Light therapy (phototherapy)
Sessions exposed to white artificial light at 3300 lux were effective in relation to the motor manifestations of PD and the emotional state of patients [41]. The use of a light source of 5000 lux was accompanied by a significant reduction in daytime sleepiness and weakness in patients with PD compared to the placebo group (light source of 300 lux) [42]. The authors attribute these effects to optimization of circadian cycles of melatonin production.
Transcranial micropolarization (TCMP)
Domestic works concerning transcranial electropolarization in PD are of interest; its use is accompanied by a decrease in hypokinesia [43]. However, in these studies there is no clear description of the criteria for inclusion of patients, and there is also no element of blinding. TCM is attracting increasing attention from foreign researchers. Thus, in a randomized placebo-controlled trial, positive effects were shown in terms of improved gait and reduction of bradykinesia, although no clear improvement was noted on the UPDRS scale [39]. Another pilot study reported significant improvements in walking with TCM [44].
A study of the use of mental relaxation and auto-training techniques did not show effectiveness in PD [34]. The effectiveness of using general vibration (vibromassage) has also not been demonstrated [45]. The potential for disaggregation of pathological α-synuclein to treat PD using laser therapy (photoacoustic therapy) is currently being discussed, but data regarding its efficacy and safety are currently lacking.
Some studies have shown the positive effect of acupuncture (acupuncture, apitherapy), but meta-analyses do not confirm these data, primarily due to the small number of participants. A significant limitation of these studies is the difficulty of reproducing the technology, which can vary significantly between two different specialists. Currently, there are a number of single-center, simple randomized and blinded studies in well-known acupuncture centers (mainly in South Korea and China), which have demonstrated a positive effect in small groups of patients. It should be noted that the vast majority of studies were conducted using acupuncture as an adjunctive treatment to levodopa therapy. There are interesting results from studies assessing not so much clinical endpoints as changes during instrumental studies. Thus, according to single-photon emission computed tomography data, patients undergoing acupuncture (in combination with levodopa therapy) compared with patients undergoing drug therapy alone had a significantly greater regional change in blood flow with the same activity of dopamine transporting systems [46]. Similar results regarding brain metabolism were obtained using positron emission tomography [47]. In our opinion, there is no definitive answer regarding the effectiveness of acupuncture in PD. It can only appear with proper standardization of acupuncture techniques and multicenter studies using standard methods.
Thus, the search for optimal methods of non-drug treatment for PD currently continues with varying degrees of success. The level of evidence is gradually increasing. Continuing research and developing unified approaches to non-drug therapy for PD is of great importance, taking into account the low effectiveness of drugs for the treatment of non-motor symptoms and walking disorders. In terms of restoring walking, there are reasonable hopes for TRK methods.
There is no conflict of interest.
Patent for invention No. 2321345, registered in the State Register of Inventions of the Russian Federation on April 10, 2008.
Patent for invention No. 2281695, registered in the State Register of Inventions of the Russian Federation on August 20, 2006.
Patent for invention No. 2304997, registered in the State Register of Inventions of the Russian Federation on August 27, 2007.
Patent for invention No. 2527170 “Method of treating patients with parkinsonism.”
Complications
Restriction of muscle function in Parkinson's disease inevitably leads to a decrease in the intensity of blood flow and a decrease in the level of metabolism. Already in the middle stages of the development of the disease, the risk of blood clots and the development of dangerous conditions increases significantly:
- ischemic or hemorrhagic stroke;
- myocardial infarction, angina pectoris, heart failure;
- pulmonary embolism.
Bedridden patients often face severe complications associated with a recumbent position:
- congestive pneumonia;
- aspiration pneumonia (due to impaired swallowing, food enters the respiratory tract);
- bedsore infection and sepsis.
Most patients experience apathy and severe depression, which lead to suicidal thoughts. Some patients carry out plans and commit suicide.
Correctly selected treatment significantly reduces the risk of complications, which is why it is important to consult a doctor in a timely manner.
Mechanism of astrocyte transformation
PTB1 as a target for astrocyte reprogramming [14]. This protein is synthesized in astrocytes and inhibits differentiation into neurons. Decreased production of PTB1 causes the production of its neural variant nPTB1 .
First, the scientists transformed astrocytes in vitro. Astrocytes were isolated from mouse midbrain and cortex and from human cortex. The researchers used a hairpin RNA to the Ptbp1 gene, which encodes the PTB1 protein. Hairpin RNAs work on the principle of RNA interference: they interact with the messenger RNA of a specific gene and cause its degradation, resulting in the cell's inability to produce protein. The level of PTB1 in astrocytes dropped, which led to the transformation of astrocytes into neurons.
The researchers then moved on to in vivo experiments: They depleted the PTB1 protein in the mouse brain. Symptoms of Parkinson's disease in mice were induced using a toxic dopamine analogue, 6-OHDA, which causes the death of dopaminergic neurons. Qian and colleagues used transgenic mice producing cre recombinase. This allowed the viral vector to be targeted directly to astrocytes. To make sure that the virus reached its target, a red fluorescent protein gene was inserted into it. The viral vector contained a small hairpin RNA (shPTB) that blocked the Ptbp1 gene. This strategy also led to the conversion of astrocytes into neuronal cells and the restoration of motor activity (Fig. 3).
Figure 3. Left: Mouse astrocytes. Right: neurons obtained through astrocyte reprogramming.
One-Time Treatment Generates New Neurons, Eliminates Parkinson's Disease in Mice
Prevention
Prevention of Parkinson's disease includes:
- proper nutrition with a minimum amount of preservatives and artificial additives, a sufficient amount of vitamin D, omega-3, antioxidants;
- regular physical activity to prevent physical inactivity;
- adequate sleep of at least 8 hours a day, adherence to a work and rest schedule;
- minimizing stress and fatigue;
- quitting smoking and drinking alcohol;
- regular medical examinations, compliance with all doctor’s recommendations;
- timely contact a specialist if any suspicious symptoms appear.
Make an appointment
Treatment at the Energy of Health clinic
Neurologists at the Energy of Health clinic will come to the rescue at any stage of Parkinson’s disease. We offer comprehensive treatment in accordance with modern standards:
- selection of drug therapy;
- physiotherapeutic procedures: magnetotherapy, laser treatment, etc.;
- therapeutic massage courses;
- Exercise therapy directly in the clinic;
- training in physical exercises for exercise at home;
- organization of sanatorium-resort treatment if indicated;
- training relatives in the rules of caring for the sick;
- work with a psychologist and psychiatrist if necessary.
Treatment with folk remedies
In conclusion, the so-called “folk remedies for Parkinson’s disease” should be mentioned. Nowadays, various non-drug methods of combating this disease are gaining popularity, including thanks to some unscrupulous media sources, including pseudoscientific sites.
When deciding to use all these recipes, decoctions, tinctures and mixtures, there are several points to consider. First, it should be remembered that none of the herbal remedies have an evidence base. And secondly, there is such a simple method as statistics. Before the invention of modern methods of treating Parkinson's disease, patients, as mentioned above, usually lived no more than 10 years. Whereas now life expectancy does not differ significantly from normal. All this suggests that no “wisdom of ancestors” or “centuries of experience” has had or can have any effect in the treatment of this disease. While many recipes can cause harm.
Remember that therapy for Parkinson's disease, methods for selecting tablets for treatment and their dosages are within the competence of neurologists. Don't self-medicate!
Advantages of the clinic
The Health Energy Clinic has experienced doctors, skilled nurses and modern equipment for diagnosing and treating various diseases. We offer:
- diagnostics using instrumental, functional and laboratory studies;
- consultations with experienced specialists in various fields;
- obtaining the opinion of foreign colleagues if necessary;
- individual selection of treatment;
- complex therapeutic regimens using medications, physiotherapy and other methods;
- extensive screening programs for early diagnosis of diseases;
- detailed consultations on disease prevention;
- any certificates and conclusions.
Parkinson's disease begins very slowly, but it is almost impossible to stop the process. If you or your family have suspicious signs, do not delay contacting a doctor. Sign up for diagnostics at the Energy of Health clinic.