Toxoplasmosis is a human disease caused by the microscopic single-celled parasite Toxoplasma gondii. It has a wide variety of clinical manifestations, mainly affecting:
- Nervous system.
- Eyes.
- Skeletal and cardiac muscles.
- Lymphatic system.
The pathogen is very common in nature; it is carried by several hundred species of animals and birds, including domestic ones. The parasite easily penetrates the body, but often does not lead to the development of an acute infection. The disease usually develops in people with a reduced immune response - patients with AIDS and other immunodeficiency conditions, when taking immunosuppressants.
Pathogens of the disease, its sources
The most dangerous is intrauterine infection, which often ends in miscarriage or severe congenital pathologies. For this reason, testing for toxoplasmosis must be carried out in preparation for pregnancy and bearing a child.
According to statistics, toxoplasma is found in 25-80% of the world's population. In some regions, the infection rate reaches 95%.
Toxoplasma is an intracellular parasitic organism that can exist in three forms:
- Tachyozoites. They have a crescent shape and a size of 2-4 microns. A characteristic feature is that with special staining (Romanovsky-Giemsa), the nucleus becomes red, and the cytoplasm becomes blue-gray. In this form, the parasite penetrates the cells of the immune system (macrophages, phagocytes), in which it quickly multiplies. After cell death, tachyozoites are released and infect healthy macrophages.
- Bradiozoites (pseudocysts). This is a protective form of Toxoplasma, in which most of the life activity of the pathogen occurs during a normal immune reaction on the part of the infected organism. The shape of the bradiozoite cell is elongated, the nucleus is displaced towards one of the ends. Toxoplasma in this form often forms tissue pseudocysts consisting of many parasitic cells covered with a common protective membrane.
- Oocysts. This form of Toxoplasma is found only in the intestinal epithelial cells of the domestic cat and its wild relatives. Formed as a result of sexual reproduction of the parasite inside the cells of the animal. The immature oocysts are then released into the environment in feces. Given sufficient temperature and an influx of fresh oxygen, the oocysts mature within 2-7 days. After maturation is completed, oocysts become capable of infecting intermediate hosts of Toxoplasma, including humans.
The main source of infection is representatives of the cat family, the overwhelming majority being domestic cats. The mechanism of transmission of the parasite is fecal-oral. Implemented through:
- Consumption of insufficiently thermally processed meat products (especially pork and lamb).
- Through poorly washed vegetables, herbs, fruits.
- Failure to comply with personal hygiene rules (unwashed hands).
Rarely, direct infection with Toxoplasma through microdamage to the skin is possible. The parasite is rarely transmitted from mother to child. On average, one infection per 2-3.5 thousand pregnant women. It is most likely that the fetus will become infected with Toxoplasma if during pregnancy the pregnant woman’s body encounters the parasite for the first time.
Susceptibility to Toxoplasma is extremely high; single oocysts are sufficient for infection. Young people are most susceptible; in older people (after 60 years), the parasite is detected much less frequently.
Prevention
Compliance with basic rules of personal hygiene is the key to health. Since pets often become carriers of parasites and infections, you should wash your hands thoroughly after contact with them and avoid interaction with street cats. In addition, prevention of brain toxoplasmosis includes the following recommendations:
- do not try poorly fried or raw meat;
- When working with someone else's blood, ensure sterility;
- check the quality of transfused blood;
- rinse greens, fruits, vegetables well;
- disinfect your hands after cleaning your pet's litter box or working with soil;
- thoroughly wash kitchen utensils after cutting meat;
- destroy parasites in the house that can carry infectious agents (flies, cockroaches).
Acquired toxoplasmosis, symptoms
The majority of those infected have no obvious clinical symptoms; the disease immediately enters the latent carrier phase. The incubation period ranges from 1 to 3 weeks.
When a pronounced clinical picture appears, the symptoms increase slowly. Usually the disease begins asymptomatically with enlargement of regional lymph nodes (inguinal, axillary, cervical). Lymph nodes are elastic, painless, patients do not show any complaints during this period.
Then the body temperature rises to 38-39 degrees, headaches appear, and symptoms of acute gastroenteritis develop. In particularly severe cases of toxoplasmosis, fever begins suddenly, and body temperature can rise to 40 degrees or more. Intense sweating, severe intoxication, abdominal pain and macular rash are observed.
After the first week of development of the infectious process, the spleen and liver become enlarged. Aching pain may occur in large muscle groups of the lower and upper extremities. Every fifth person develops chorioretinitis during this period, which manifests itself in loss of areas of the visual field.
Starting from the second week, the symptoms of gastrointestinal tract damage begin to fade. Symptoms of enteritis decrease and quickly disappear, and overall intoxication of the body decreases. At the same time, the lesion develops:
- Musculoskeletal system. Pain in the limbs and joints increases, and mobility and fine motor skills may be impaired.
- Reticuloendothelial. Manifests itself as hepatolienal syndrome, mesadenitis.
- Cardiovascular. Heart rhythm disturbances and symptoms of myocarditis or pericarditis often develop.
At 3-4 weeks, the disease ends with the attenuation of all manifestations and the transition of toxoplasmosis to asymptomatic carriage. When exposed to negative factors that weaken the immune system, the disease may manifest with the development of the above-described clinical picture, which again enters the latent phase.
Frequent relapses of acute toxoplasmosis, especially against the background of immunosuppressive factors, can lead to serious complications. More common:
- Myocardial dystrophy.
- Psychoneurological pathologies.
- Decrease in intelligence.
- Atrophy of the optic nerve up to complete blindness.
- Chronic fatigue syndrome.
Acute toxoplasmosis in women can lead to menstrual irregularities, miscarriages and other pathologies of the reproductive system.
Chronic acquired toxoplasmosis is a fairly rare phenomenon and is considered an AIDS-associated form of the disease. It occurs with periodic exacerbations; complications from the central nervous system often develop in the form of encephalitis and damage to the visual organs.
The most severe consequence of chronic toxoplasmosis is a generalized infection during which multiple organ failure quickly develops, sometimes this complication ends in the death of the patient.
Causes of the disease
The pathology is provoked by Toxoplasma, a protozoan that is part of the sporozoan class. Externally, the parasite resembles an orange slice or a crescent moon. The unicellular organism has a complex development cycle; the main host is considered to be cats, in whose body it reaches sexual maturity. Infection of an animal is associated with eating sick individuals: rodents, birds.
When Toxoplasma enters the cat’s gastrointestinal tract, it actively multiplies, and new parasites are excreted along with the feces. Humans become infected through contact with cat feces; they are intermediate hosts.
Upon penetration into the human body, the protozoan moves along with the bloodstream and is localized in the organs of vision, lymph nodes, and brain tissue. There, Toxoplasma destroys cells and forms cavities. After its death, calcifications are formed in the body, consisting of the body of the parasite and calcium salts.
Several strains of the pathogen are isolated, some do not provoke the appearance of symptoms, others lead to the death of animals. The parasite is not resistant to external factors and quickly dies at high temperatures or in chemical compounds.
Congenital toxoplasmosis occurring during pregnancy
Occurs when the parasite penetrates the placental barrier and infects the fetus. In most cases, it occurs during primary infection during pregnancy, less often - during relapse of toxoplasmosis associated with decreased immunity. The main risk group is women who were not infected with toxoplasma before pregnancy. If, as a result of contact with the pathogen, the disease manifests itself during pregnancy, the likelihood of developing congenital toxoplasmosis is quite high.
The survival rate of children with intrauterine infection depends on the period at which it occurred:
- If infected in the first trimester, the chance of fetal survival is 15%
- On the second – 30%.
- On the third – 60%
Even if pregnancy is successfully completed, an extremely high degree of development of congenital pathologies and congenital toxoplasmosis remains. The disease is severe, especially if infection occurs in the early stages. A characteristic tetrad of pathologies develops:
- Hydrocephalus.
- Bilateral retinochoroiditis.
- Delayed psychophysical development.
- Cerebral calcifications.
The prognosis for congenital toxoplasmosis is unfavorable; in most cases, the disease ends in the death of the newborn or severe disability. Even if, after intrauterine infection, an acute clinical picture does not arise, such children are at risk of developing:
- Mental deficiency.
- Epilepsy.
It is possible to develop many other pathologies that appear months and years after birth. For this reason, an acute form of toxoplasmosis that occurs during pregnancy is an indication for abortion even in the later stages. During pregnancy, it is necessary to undergo a lot of tests that help identify the presence of not only toxoplasmosis, but also CMV infection.
How to treat toxoplasmosis?
For each patient, the course of treatment for toxoplasmosis is prescribed strictly individually and lasts until all clinical manifestations of the disease that worsen the person’s quality of life are eliminated.
In order to cope with the disease, antiparasitic drugs are required, which are most often prescribed in combination:
- Pyremethamine;
- Spiramycin;
- Azithromycin;
- Daraprim;
- Sulfadimezin;
- Clindamycin.
Medicines are used in courses at certain intervals over a long period of time. Hormonal drugs (glucocorticoids) are prescribed to patients with damage to the visual organs and nervous system.
Infection with this disease poses a serious threat to persons suffering from immunodeficiencies of various origins (for example, HIV infection) or receiving suppressive drug therapy. Such patients have a significantly worse prognosis, are difficult to cure, and require special treatment regimens.
In case of primary infection of a pregnant woman under 17 weeks, abortion is recommended for medical reasons; not only the parasite itself is dangerous for the fetus, but also the medications used to combat it. If this occurs after this period, the patient is treated with antiparasitic drugs.
Indications for examination
Analysis is most often prescribed in two cases:
- When planning pregnancy as part of a standard laboratory diagnostic package for TORCH infection.
- If you suspect toxoplasmosis and have certain symptoms.
Laboratory diagnostics are also used to identify symptoms of acute toxoplasmosis in adults or children.
In general medical practice, this test is prescribed if patients exhibit specific symptoms (visual impairment, seizures), as well as for HIV-infected patients.
Differential diagnosis
It is carried out with diseases whose symptoms are similar to acute and chronic forms of toxoplasma infection. These include:
- Infectious mononuclease.
- Mycoplasmosis.
- Chlamydia.
- Cytomegaly.
- Tuberculosis.
It is also necessary to exclude oncological pathologies and systemic diseases (rheumatism, lymphogranulomatosis). The final diagnosis is established after receiving the results of specific serological tests and PCR. There are many laboratory methods for detecting specific antibodies to Toxoplasma. These include:
- ELISA. Linked immunosorbent assay.
- RNIF. Indirect immunofluorescence reaction. Becomes positive from the first week of the disease. High antibody titers can last up to 15 years.
- RSK. Complement fixation reaction. It becomes positive from the 10-14th day of disease development and persists for 2-3 years.
Clinic, diagnosis and treatment of toxoplasmosis
Toxoplasmosis is a widespread zoonotic parasitic infection, characterized by polymorphism of clinical manifestations and significant variability in the course of the process: from healthy, asymptomatic carriage to severe, lethal forms of the disease.
Etiology
The causative agent of toxoplasmosis, Toxoplasma gondii, belongs to the phylum Protozoa, subphylum Sporozoa, order Eucoccidia. T.gondii is an intracellular parasite, 4–7 µm in size.
In the human body, T.gondii can parasitize in the form of proliferative forms—endosites, in the form of pseudocysts, and in the form of true tissue cysts. In cats and other representatives of the feline family, toxoplasmosis can also be present in the intestines in the form of oocysts, which, when excreted with feces into the external environment, remain viable and invasive for 1.5–2 years. Toxoplasma cysts found in meat and meat products can remain viable at a temperature of 2–5°C for up to a month, but quickly die when heat treated or frozen at –20°C. The least resistant to environmental factors are endosites, which remain viable outside the body from 30 minutes to several hours.
Epidemiology
Toxoplasma or traces of its presence have been found in more than 200 species of mammals and 100 species of birds. The prevalence or infection rate of the population of the Russian Federation with Toxoplasma is on average about 20.0%. Infection rates are higher in regions with warm climates; among persons of a number of professional groups (epidemiological observations have proven an increased infection and incidence of toxoplasmosis in people who have professional contact with sources of toxoplasmosis infection (workers of meat processing plants and fur farms, livestock breeders, veterinarians, etc.). The infection rate in women is usually 2–3 times higher than men.
The incidence of toxoplasmosis is many times lower than infection rates, but diagnostic difficulties, despite mandatory registration, do not allow us to judge the true level of infection.
Susceptibility to toxoplasmosis is almost universal. The spread of infection is widespread in the form of carriage and sporadic diseases.
The main source of infection for toxoplasmosis is stray, wild and domestic cats, in whose bodies the pathogen goes through a full development cycle (tissue and intestinal) and is excreted in the form of oocysts with their feces. Cats shed the pathogen for an average of three weeks from the moment of infection. During this time, up to 1.5 billion toxoplasma enters the environment.
The main transmission factor is raw or insufficiently heat-treated meat, meat products with toxoplasma cysts in it. Additional factors for transmission of infection include poorly washed greens, vegetables, fruits (from the ground), dirty hands with pathogen oocysts on them.
The main and most common route of transmission of infection is oral; much less often, human infection can be carried out by transplacental (blood transfusion), percutaneous and transplantation routes.
A person with toxoplasmosis does not pose an epidemiological danger either to others or to medical personnel, which makes it possible to treat these patients both on an outpatient basis and in any somatic hospital.
Immunity. Immunity for toxoplasmosis is non-sterile and infectious. The immune state of the body is preserved simultaneously with the presence of the pathogen in the body, most often in the form of cysts. Antigenic metabolites produced by cysts maintain a certain level of humoral immunity and also cause the development of delayed-type hypersensitivity.
Pathogenesis
The pathogens released from cysts or oocysts invade the epithelial cells of the small intestine, where they multiply, forming a primary affect and then penetrating into the regional lymph nodes, and from them, with the lymph flow, into the blood. Dissemination of the pathogen leads to damage to a wide variety of organs and tissues.
Toxoplasma has a cytopathogenic effect on the cell, and inflammatory granulomas are formed at the sites of their penetration. Necrosis develops, in place of which lime salts fall out—calcifications characteristic of toxoplasmosis are formed. The degree of damage to a particular organ further determines the clinical symptoms of the disease.
The formation of immunity leads to the disappearance of the pathogen from the blood, and its reproduction in cells stops. True tissue cysts are formed, which can remain intact in the body for a long time, for decades (carriage of Toxoplasma). In humans, infection, as a rule, has a benign course, without the development of septic conditions.
Clinic
Most infected people have no clinical manifestations of the disease. In the vast majority of cases of toxoplasmosis, a healthy carriage of the parasite is observed, accompanied by a consistently low level of specific antibodies in the blood. Carriage does not require any therapeutic measures, and the carrier should be regarded as a practically healthy person.
At the same time, clinically pronounced variants of the course of the infection are possible, requiring careful diagnosis and specific treatment.
According to the nature of its course, acquired toxoplasmosis is divided into acute and chronic. In addition, depending on the duration of the disease and the severity of clinical symptoms, a subacute as well as inapparent (subclinical) course of the infection is possible, which is characterized by certain dynamics or a high level of specific antibodies in the blood, in the absence of clinical manifestations of the disease. Thus, the most convenient for practical healthcare, from our point of view, is the following classification of acquired toxoplasmosis: acute, subacute, chronic, inapparent and carriage.
The incubation period for toxoplasmosis lasts on average up to 2 weeks, although sometimes it can last up to several months. The disease, as a rule, begins gradually: general weakness, malaise, muscle pain, chilling appear, performance decreases, and the temperature rises to low-grade levels. The lymph nodes become enlarged: cervical, occipital, and less commonly, axillary and inguinal. Lymph nodes are soft, slightly painful on palpation. The size of the nodes is 1–1.5 cm, they are not fused with the surrounding tissues, do not form conglomerates, and the skin over them is not changed. Sometimes the mesenteric lymph nodes are significantly enlarged, which can simulate the picture of an acute abdomen.
An acute onset, with a rise in temperature to 38°C and above, involving in the process, in addition to the lymphatic, nervous system, internal organs, muscle tissue, and organs of vision, is observed much less frequently. Patients may develop encephalitis, myocarditis, myositis, uveitis (chorioretinitis). In some cases, a short-term roseolous-papular rash and hepatolienal syndrome are observed. In patients with immunological disorders (especially in patients with AIDS), pneumonia, enterocolitis, severe central nervous system disorders of a cystic-necrotic nature, and a septic condition may develop.
Chronic toxoplasmosis is a long-term process with a general infectious syndrome and the presence of organ lesions of varying severity. The most characteristic signs of chronic toxoplasmosis are prolonged low-grade fever, intoxication and asthenia; fever can last for months, with slight temperature fluctuations, sometimes short periods of apyrexia, which is not amenable to conventional means of therapy. A common manifestation of toxoplasmosis is a generalized enlargement of the lymph nodes - occipital, cervical, inguinal and others.
Damage to the central nervous system in chronic toxoplasmosis most often occurs in the form of cerebral, basal arachnoiditis; Hypertensive and diencephalic syndromes develop, vegetative-vascular disorders are detected, and episyndrome is noted. Sluggish myocarditis, myocardial dystrophy and myositis may be observed. Women may have specific inflammatory diseases of the genitals - toxoplasma salpingo-oophoritis (adnexitis); primary and secondary infertility develop.
Eye damage, both in acute and chronic acquired toxoplasmosis, occurs as posterior uveitis (focal chorioretinitis). Chorioretinitis is usually central, bilateral, and recurrent. The development of conjunctivitis, keratitis, iridocyclitis, central exudative retinitis, optic neuritis with outcome in dystrophy, complicated myopia is possible.
X-ray examination of a patient with toxoplasmosis allows in some cases to reveal the presence of calcifications in the soft tissues of the brain. Calcifications are usually small, multiple, round in shape, often with smooth contours.
In both acute and chronic toxoplasmosis, there are no special changes in the peripheral blood. Leukocytosis observed at the onset of the disease is replaced by normocytosis, relative lymphocytosis is detected, ESR is within normal limits.
It should be emphasized that with chronic toxoplasmosis there is no isolated damage to any one organ or system; we can talk about predominant organ damage against the background of the general process.
Diagnostics
Differential diagnosis. Toxoplasmosis should be differentiated from infectious mononucleosis, benign lymphoreticulosis, tuberculosis, brucellosis, listeriosis, mycoplasmosis, chlamydia, cytomegaly, herpes and a number of other bacterial, viral and parasitic infections. Oncological and systemic diseases should be excluded (for example: lymphogranulomatosis, rheumatism, etc.).
Laboratory diagnostics. For laboratory diagnosis of toxoplasmosis, serological methods are most often used: complement fixation test (CFR), indirect immunofluorescence reaction (IRIF), enzyme-linked immunosorbent assay (ELISA). The diagnosis is confirmed by a significantly increasing dynamics of the indicators of these tests, their high level or the presence of IgM class antibodies.
When giving a clinical assessment of the results of a serological examination of a patient, it is necessary to take into account that RNIF becomes positive from the first week of the disease and reaches its maximum values (1:1280–1:5000) usually by the second to fourth month of the disease; in low titers it can persist from a year to 15 years. RSC becomes positive from the second week of the disease, reaches maximum values (1:160–1:320) by the second to fourth month of the disease, but after 2–3 years it can disappear or decrease to values of 1:5–1:10. The interpretation of ELISA results is more objective, since it is focused on the WHO International Standard. Positive results may be indicated by indicators expressed in optical density units (OD ≥1.5); enzyme-linked immunosorbent units (EIU) ≥60; International units (IU) ≥125 and antibody titres (TA) ≥1:1600. The basic principle of serological diagnostics—the dynamics of the increase in indicators—is also applicable to this method. Of significant importance in the diagnosis of toxoplasmosis, especially in differentiating acute and chronic processes, is the determination of immunoglobulin classes, in particular IgM class antibodies. Toxoplasma infection can be reliably diagnosed only by comparing the results of serological reactions over time. Antibodies of all classes increase significantly by the end of the second - beginning of the third week from the moment of infection and reach a diagnostic level.
The diagnosis of toxoplasmosis, in the presence of an appropriate clinic, can be made with a positive serological conversion, when the second serum test becomes positive. When treating patients with already established positive reactions to toxoplasmosis, the question of diagnosis and activity of the infectious process can be resolved in the dynamics of serological studies. With fresh infection and disease, serological reactions are often positive with high titers of antibodies, and specific IgM is detected. When latent toxoplasmosis is reactivated, the IgM concentration may increase, but in this case the severity of the IgM response will be much less than during the primary infection. Positive RNIF in a low titer may indicate chronic toxoplasmosis or asymptomatic carriage of the pathogen. It should be noted that in ocular pathology, the presence of a fresh focus of inflammation, even with low antibody titers, indicates toxoplasmosis. In case of lymphadenopathy, even with high antibody titers, the diagnosis of toxoplasmosis is made only after histological examination of the lymph nodes and consultation with an oncologist. Based on the results of a single study, it is impossible to establish the duration of the infectious process and the exact correspondence to one or another of its stages, whereas this question is fundamental for assessing the risk of intrauterine infection of the fetus. Women who have had an infection before conception and women with chronic toxoplasmosis are practically immune from the risk of intrauterine infection of the fetus, while pregnant women infected in the first and early second trimester of pregnancy constitute the main risk group. However, it must be remembered that identifying and confirming the presence of IgM antibodies is not a clear indicator of termination of pregnancy. It is necessary to use additional methods to reduce the risk of inaccurate diagnosis.
Formation of diagnosis. When forming a detailed diagnosis of toxoplasmosis, the following should be indicated:
- form of toxoplasmosis (acquired, congenital);
- the nature of the process (acute, subacute, chronic, inapparent);
- organ or systemic pathology;
- severity of the current.
For example: acute acquired toxoplasmosis, lymphadenopathy, mild course; chronic acquired toxoplasmosis with primary damage to the eyes, chorioretinitis without exacerbation; pregnancy 24–26 weeks, inapparent toxoplasmosis.
When forming a diagnosis of toxoplasmosis, it is inappropriate to base the diagnosis of toxoplasmosis only on systemic or organ pathology (lymphadenopathic, cerebral, myocardial, ocular form, etc.), because toxoplasmosis must be considered as a general process involving many organs and systems.
When toxoplasmosis is excluded and a patient with positive reactions to toxoplasmosis is given another diagnosis, the medical history should also note the existing carriage of toxoplasma.
Treatment
The choice of treatment tactics depends on the form and nature of the disease, the severity of clinical symptoms, the severity of the course, the presence of complications and the predominant organ-systemic lesions.
The absolute indications for treatment are acute and subacute toxoplasmosis. Treatment of chronic toxoplasmosis is carried out depending on the severity of clinical symptoms and the nature of the predominant lesions. Inapparent toxoplasmosis detected in pregnant women also requires treatment.
The drugs Fansidar, Rovamycin and Biseptol are prescribed. Fansidar contains sulfadoxine 500 mg and pyrimethamine 25 mg. Etiotropic therapy consists of 2–3 cycles. Prescribed 1 tablet 1 time in 3 days (No. 8 tablets). Folic acid is prescribed between cycles. In case of intolerance to drugs of the pyrimethamine group, Rovamycin is prescribed, 1 tablet of which contains 3 million IU of spiromycin. Prescribe 3 million IU 3 times a day with a seven-day break. Rovamycin is well tolerated by patients, lack of drug interactions, and high efficiency allow it to be prescribed for the treatment of toxoplasmosis in all age groups.
It is possible to use combination drugs; Poteseptil (trimethoprim + sulfadimezine), Biseptol (trimethoprim + sulfamethoxazole), which are prescribed 1 tablet 2 times a day for 10 days (cycle), in the amount of 2-3 cycles (course). If these drugs are intolerant when taken orally, it is possible to prescribe Biseptol intravenously or drip: 10 ml per day, for 5 days (course). In the intervals between cycles (courses) of etiotropic therapy, folic acid is prescribed, on average up to 0.01 g per day.
If an immunodeficiency state is detected, immunotropic drugs are prescribed together with etiotropic therapy: Likopid, Cycloferon, Vitamedin-M, as well as natural calf thymus hormones and their synthetic analogues: Taktivin, Timamin, Thymogen, Dekaris.
Systemic enzyme therapy (SET) drugs, in particular Wobenzym and Phlogenzyme, are also used in complex therapy. To preserve the intestinal microbiocenosis, it is recommended to prescribe pro- and prebiotics.
Treatment and further follow-up of patients should be carried out by specialists depending on the nature of the prevailing pathology - infectious disease doctors, neurologists, ophthalmologists, obstetricians-gynecologists, etc. Hospitalization is carried out in a hospital of the appropriate profile (infectious diseases, neurological, ophthalmological, obstetric -gynecological, children's, etc.). This is due to the peculiarities of organ pathology, the specifics of the examination and the prescription of additional treatments.
Clinical examination
Dispensary observation is carried out by doctors according to the profile of the predominant pathology, in each individual case individually. After suffering from acute (inapparent) toxoplasmosis, patients are examined and examined every 3–4 months for a year, then 1–2 times a year. Patients with chronic toxoplasmosis are consulted 2 times a year.
Prevention
Prevention of acquired toxoplasmosis includes: eating only well-heat-treated meat products; eliminating the habit of tasting raw minced meat or raw meat; eating clean washed vegetables, herbs and fruits (from the ground); thorough hand washing after handling raw meat, raw meat products, after working in the garden, or for children - after playing on the playground, especially in the sandbox; fight against stray cats; treatment of patients with toxoplasmosis in domestic cats, prevention of infection of the latter. Specific prevention of toxoplasmosis has not been developed.
Diagnosis, clinical picture and treatment of toxoplasmosis in pregnant women
Diagnosis of toxoplasmosis in pregnant women includes the entire range of necessary clinical, paraclinical and special (immunobiological) studies that are used to diagnose toxoplasmosis in general.
A mandatory condition for examining a pregnant woman for toxoplasmosis should be a consultation with an infectious disease specialist to confirm or exclude a current infectious manifest or asymptomatic (inapparent) toxoplasma process. If the diagnosis is confirmed and the need for treatment is carried out, the latter is carried out by an obstetrician-gynecologist either on an outpatient basis (in a antenatal clinic) or in an obstetrics-gynecology hospital (maternity hospital). Considering the epidemiological safety of patients with toxoplasmosis for others, a pregnant woman with an uncomplicated obstetric history, but with a diagnosis of toxoplasmosis, can be hospitalized for examination, treatment and delivery in any (physiological) department of the maternity hospital. Pregnant women with a burdened obstetric history and diagnosed with toxoplasmosis are hospitalized in pregnancy pathology departments.
Screening of pregnant women for toxoplasmosis should be carried out by antenatal clinics when the pregnant woman first visits there. When conducting this examination, the obstetrician-gynecologist must remember that in our country, depending on the region, the percentage of infected women of childbearing age is on average 20–30%, i.e. every third of them can react positively to toxoplasmosis. As a rule, pregnant women with positive immunological reactions are healthy carriers of the pathogen and do not require any therapeutic, much less surgical measures. These women have virtually no complaints or objective manifestations of infection. Levels of specific antibodies remain stably at the same, usually low levels; there are no specific antibodies of the IgM class. 70–80% of women are free from infection and react negatively to toxoplasmosis. These women represent a “risk” group for congenital toxoplasmosis, since 0.5–1% of them become infected with toxoplasmosis during pregnancy. Of women primarily infected during pregnancy (high-risk group), 30–40% transmit the infection to the fetus. Consequently, non-immune (immunonegative) women are subject to dispensary observation for the prevention of congenital toxoplasmosis and dynamic examination (once every 1-2 months) during pregnancy in order to identify a fresh infection.
Clinical manifestations of toxoplasmosis in pregnant women do not have any significant differences. Acute toxoplasmosis is accompanied by an increase in temperature to febrile (usually subfebrile) levels, lymphadenitis is detected (usually posterior cervical and occipital), and possible disorders of the central nervous system, internal organs, eyes and muscles. When women become infected shortly before pregnancy or in the early stages of pregnancy, Toxoplasma may damage the fetal egg, usually leading to miscarriage. The obstetrician-gynecologist also needs to remember about the possible inapparent (asymptomatic) course of acute toxoplasmosis in women during pregnancy, when the development of the disease is recorded either by the significantly increasing dynamics of the level of specific antibodies, or by the detection of immunoglobulins of the IgM class in ELISA, which dictates the need for serological control (screening). ) for uninfected pregnant women throughout pregnancy.
Chronic toxoplasmosis in pregnant women is characterized by a general infectious syndrome (low-grade fever, generalized lymphadenopathy, chilling, decreased ability to work, etc.) with possible predominant organ damage from the internal organs, eyes, central nervous system and genitals.
The indication for prescribing etiotropic anti-Toxoplasma therapy to pregnant women is acute, subacute and inapparent toxoplasmosis. Treatment of chronic toxoplasmosis should be carried out strictly according to clinical indications either before or after pregnancy.
In the absence of complaints and clinical indications, women who have had toxoplasmosis before pregnancy do not need treatment. These women are regarded as practically healthy individuals who do not require special medical supervision.
Thus, the question of the time of infection of a pregnant woman is practically important: long before, immediately before or during pregnancy. The duration of infection is determined based on anamnesis and a comprehensive examination of the woman (cordocentesis and amniocentesis). Treatment of pregnant women should be carried out no earlier than 12–16 weeks of pregnancy (from the second trimester of pregnancy). Treatment is usually carried out with Rovamycin, Fansidar.
Rovamycin is prescribed in a daily dose of 3 million 2 times a day, the duration of administration is 7 days, the number of cycles is 2, the break between cycles is 1 month.
The concentration of Rovamycin in the placenta is 5 times higher than in the blood serum, which ensures cure. Rovamycin is the first macrolide used to treat toxoplasmosis in pregnant women. Rovamycin is well tolerated by patients, lack of drug interactions, and high efficiency allow it to be prescribed for the treatment of toxoplasmosis in all age groups.
Fansidar is prescribed 1 tablet 1 time every 3 days (No. 8 tablets). The break between cycles is one month. 3 such cycles are prescribed.
Considering the possible inhibition of hematopoiesis under the influence of etiotropic drugs, it is recommended to prescribe folic acid in medium therapeutic doses, as well as conduct general blood and urine tests.
In practical work, the question of indications for artificial termination of pregnancy during toxoplasma infection and recommendations for subsequent pregnancies is of particular importance.
Summarizing all of the above, it should be emphasized that only when infected in the first trimester of pregnancy and in the presence of clinical and immunological signs of acute acquired or inapparent toxoplasmosis, when the risk of giving birth to a child with gross organic lesions of the central nervous system and visual organs is greatest, the question of terminating the pregnancy for medical reasons can be raised ! Women infected during the second and third trimesters of pregnancy are subject to treatment. Termination of pregnancy for medical reasons is not indicated for women with chronic toxoplasmosis, and even more so with carriage of the pathogen, since in these cases there is no danger of infection of the fetus, because even an exacerbation of the process in pregnant women does not lead to repeated parasitemia and, therefore, to damage to the placenta, but through her and the fetus.
When giving recommendations for subsequent pregnancies, it is necessary to take into account that the same woman can have a child with congenital toxoplasmosis only once in her life (due to acute acquired or inapparent toxoplasmosis during pregnancy). During subsequent pregnancies, this woman may not be afraid of giving birth to a child with toxoplasmosis.
Prevention of congenital toxoplasmosis
Prevention of congenital toxoplasmosis should take into account the fact that only primary infection of a woman during pregnancy can lead to infection of the fetus. As mentioned above, out of the total number of non-immune women, up to 1% of pregnant women become infected during pregnancy. At the same time, infection of fetuses occurs in only 30–40% of them. According to the literature, the number of newborns with congenital toxoplasmosis is 1–8 per 1000 live newborns. Most often, the process in a child is asymptomatic, although the manifestation of the disease in the future cannot be ruled out. This can occur under the influence of immunosuppressive factors during the period of development of immunity, i.e. during the first 5–7–10 years of life.
Optimally, prevention of congenital toxoplasmosis should include screening women of childbearing age for toxoplasmosis before or, in extreme cases, during pregnancy. This examination is necessary to identify among them those who react negatively to this disease, i.e. non-immune women. The latter constitute the “risk” group for possible primary infection during pregnancy.
Non-immune pregnant women must be taken for clinical observation and examined for toxoplasmosis, but with the help of serological tests (RSK, RNIF, ELISA, etc.) throughout pregnancy, if possible, once every 1-2 months or at least 1 once every trimester.
If pregnant women at risk switch from negative serological reactions to positive ones and an increasing (3-4-fold) dynamics in the level of specific antibodies is detected, they need emergency preventive treatment. Treatment is carried out both in the manifest course of the infection and in the case of an in-apparatus course of the process. Children born to these women are subject to mandatory clinical and serological examination for toxoplasmosis and, if indicated, specific treatment. Children born to mothers with a clearly established primary infection during pregnancy are monitored until the age of 10, including regular clinical and immunological examinations in order to identify symptoms of congenital toxoplasmosis, which could be asymptomatic at birth.
Provided that at the last examination of pregnant women in the “risk” group, serological reactions remain negative, women (without clinical indications) do not need further special examination for toxoplasmosis and are dropped out of observation after childbirth. Children born to these women should be tested for toxoplasmosis only if clinically indicated. Pregnant women with negative immunological reactions are advised to strictly follow the basic rules for the prevention of toxoplasmosis.
Literature
- On the detection and prevention of toxoplasmosis in Moscow. Methodological recommendations (No. 25). M., 2007.
- Borisov B. A., Dzutseva F. K., Moroz B. V. Clinical and neurological manifestations of toxoplasma invasion. Tutorial. M., 2003.
- Bartlett J., Galant J. Clinical aspects of HIV infection. Johns Hopkins University School of Medicine. 2005–2006
F.K. Dzutseva , doctor of the highest category, head. City Center for Toxoplasmosis G. Yu. Nikitina , Candidate of Medical Sciences Yu. V. Borisenko , Candidate of Medical Sciences L. P. Ivanova *, Candidate of Medical Sciences, Associate Professor of the City Clinical Hospital named after. S. P. Botkina, *RMAPO, Moscow
Material for research
To detect specific antibodies to Toxoplasma, blood is drawn from a vein; the procedure does not require any special preparation. Any biological material is suitable for carrying out a PCR reaction - blood, saliva, tissue samples, other biological fluids (cerebrospinal fluid, urine).
If there is a need to diagnose toxoplasmosis in the fetus, blood sampling from the umbilical cord is possible. The procedure is rarely prescribed because it is associated with a high risk of complications and premature termination of pregnancy. A more gentle method is to take amniotic fluid for analysis by puncture. These methods are used when the results of specific tests on a pregnant woman with suspected toxoplasmosis do not provide sufficient clarity of the diagnosis.
Indications for the use of various laboratory tests and features of interpretation of results in different categories of subjects
Depending on the category of the patient (age group, risk group), the use of different sets of diagnostic techniques is indicated. The correct choice of diagnostic methods is especially important in cases of suspected toxoplasmosis in pregnant women.
Screening for toxoplasmosis during pregnancy
If during pregnancy a pregnant woman develops symptoms that may indicate the development of acute toxoplasmosis, it is necessary to establish the level of specific immunoglobulins using serological methods.
The enzyme-linked immunosorbent assay (ELISA) is best suited to detect the acute stage of the disease. It most accurately shows the concentration of IgM, an increase in the level of which indicates ongoing or recently present acute toxoplasmosis.
Determining IgG levels is less informative since these antibodies persist long after infection and indicate carriage rather than recent infection or exacerbation. Women who have been infected with toxoplasma before pregnancy are insured against infection of the fetus and are not at risk.
It is also important to obtain an immunological picture over time, for which specific tests are carried out at least once every 2 weeks. Studying the dynamics of changes in antibody titers allows us to establish a diagnosis with greater accuracy.
It should be taken into account that the serological picture indicating infection with Toxoplasma is not a 100% indication for termination of pregnancy. In this case, additional tests will be required by taking fetal blood from the umbilical cord and samples of amniotic fluid by puncture.
Examination of newborns and young children
Aimed at early detection of the pathogen, before the onset of the acute phase of congenital toxoplasmosis and the development of severe complications. Prescribed when there is a suspicion of Toxoplasma infection, includes the following tests:
- Isolation of the parasite by inoculating material from the placenta and umbilical cord into living mice.
- Carrying out PCR analysis of amniotic and lumbar fluids.
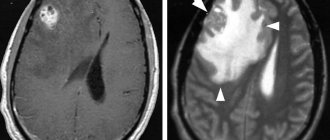
- Computed tomography or MRI of the head. Allows you to identify specific changes in the brain, for example, hydrocephalus, at an early stage.
Serological techniques are also used, but they provide only additional information. The newborn’s immune system is not active enough and is often unable to produce a sufficiently high titer of specific antibodies.
Toxoplasmosis in HIV infected people
The immunity of healthy people is able to restrain the development of infection, but toxoplasmosis with HIV can lead to serious damage to the central nervous system. A weakened immune system cannot resist the disease - asymptomatic carriage of the disease can lead to death. Problems with coordination, speech, walking, and epileptic seizures are added to the general symptoms of brain toxoplasmosis in HIV-infected people. Rapid diagnosis of the pathogen in the early stages of toxoplasma development is crucial. After stopping treatment in HIV patients, the disease may recur.
Examination of patients with HIV infection
Diagnosis consists of regular monitoring of immunoglobulin G titers using serological methods. Titration of immunoglobulin M is not informative since in most HIV-positive people the level of antibodies of this group is extremely variable.
Direct detection of the pathogen by microscopy of tissue samples or infection of laboratory animals is usually not necessary. The diagnosis can be established quite accurately through serological diagnosis and the presence of a specific clinical picture of toxoplasmosis. In HIV-positive patients it is more pronounced, which greatly simplifies the diagnosis.
Examination in Medart
At the Medart Medical Center, a full range of serological tests are performed to detect toxoplasma in the patient’s body. An immunological test to identify specific immunoglobulins is part of comprehensive STD tests intended for couples planning to have a child. It is possible to take blood and other biological fluids to determine the presence of toxoplasma and clarify the clinical picture of the disease.
High-precision modern equipment allows you to obtain accurate research results in the shortest possible time.
Advantages of Medart Medical Center:
- Qualified specialists.
- The ability to quickly obtain accurate research results.
- Affordable price.
The medical center provides a full range of services, from preliminary appointments and consultations to establishing and clarifying the diagnosis and prescribing effective treatment regimens and prevention of toxoplasmosis and other diseases.