Translation of the presentation “Anomalies and normal variants of the intracranial arteries: proposed workflow for classification and significance.”
| Congress: | ECR 2016 |
| Poster No.: | C-0199 |
| Authors: | A. Hakim1, J. Gralla1, C. Rozeik2, P. Mordasini1, F. Pult1, L. Leidolt1, E. Piechowiak1, K. Hsieh1, M. El-Koussy1; 1Bern/CH, 2Loerrach/DE |
| DOI: | 10.1594/ecr2016/C-0199 |
| DOI-Link: | https://dx.doi.org/10.1594/ecr2016/C-0199 |
Translation into Russian: Simanov V.A.
1.1. Variants of origin (discharge) of vessels
1.1.1. Common origin : two different vessels can have the same origin (discharge)
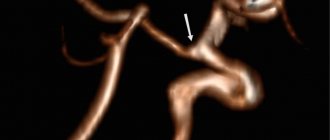
SCA/PCA: 2-22% Fig.3 :
The common trunk arises from the basilar artery, then branches into the posterior cerebral artery (PCA) and the superior cerebellar artery (SCA) [1].
PICA / AICA: common option Fig. 4 :
The anterior inferior cerebellar artery (AICA) shares a common trunk with the posterior inferior cerebellar artery (PICA) [2].
1.1.2. Funnel: 7-15% Fig.5 :
It is a funnel-shaped dilatation of the vessel at the origin. Its diameter should be no more than 3mm. It is most common at the origin of the posterior communicating artery (Pcom). A similar variant has also been described in the anterior communicating artery (Acom), ophthalmic artery and anterior choroidal artery [2].
1.1.3. Abnormal origin (discharge) due to persistent fetal circulation:
Fetal type PCA:
The posterior communicating arteries are the terminal branches of the basilar artery. During development, RCAs originate from the internal carotid artery (ICA). This variant, if it persists into the postnatal period, is called “fetal origin.” This option can be classified into two subtypes: Fig. 6
- Complete fetal PCA: 4-26% unilateral, bilateral 2-4%. PCA is entirely derived from the ICA. The P1 segment is absent, i.e., only the ICA supplies the occipital lobes [3]. In bilateral complete fetal type PCA, the basilar artery may be hypoplastic Fig. 7 .
- Partial fetal PCA: 11-29% unilateral, 1-9% bilateral. The P1 segment is still present, but is smaller or equal in diameter to Pcom, i.e. Most of the blood supply to the occipital lobes comes from the ICA[3].
Persistent dorsal ophthalmic artery (PDOA): 1.1% Fig.8, Fig.46 :
During embryonic development, the orbit is supplied with blood through the anterior and posterior rami, which originate from the ICA. Typically, the posterior branch is obliterated, while the anterior branch continues to supply the orbit. However, with this option the opposite happens. The PDOA enters the orbit through the superior orbital fissure [4].
The ophthalmic artery can also arise from other parts of the ICA, including the cavernous segment, in 8% of the population [2] Fig. 9 .
MMA from the orbital artery: 16% Fig. 10 :
During embryogenesis, the middle meningeal artery (MMA) arises from the stapedial artery. The stapedial artery gives off branches to the ECA. One of these branches is the supraorbital artery, which forms an anastomosis with the developing ophthalmic artery. Along this anastomosis, failure of segmental regression or persistence of segments that should regress leads to a number of anomalies, such as the origin of the MMA from the ophthalmic artery [2]. In this case, the foramen spinosum will be absent.
Fig. 3 TOF MRA, common trunk of PCA and SCA (red arrow) and ipsilateral dominant vertebral artery (white arrow)
Fig. 4 3D TOF MRA of the AICA (white arrow) extending caudally, supplying the PICA territory. Note the absence of PICA ipsilaterally. The contralateral PICA is present (green arrow).
Fig. 5 3D TOF MRA, funnel-shaped expansion (funnel) at the origin of Pcom (arrow).
Fig. 6 3D TOF MRA, complete fetal PCA (white arrow) with P1 segment absence on one side and partial fetal PCA (red arrow) with P1 segment hypoplasia (green arrow) on the other side. Note fenestration of the proximal part of the P1 segment (blue arrow).
Fig. 7 3D TOF, complete fetal PCAs on both sides and hypoplastic basilar artery
Fig. 8 Persistent dorsal ophthalmic artery: MIP (a) and 3D TOF MRA (b) show the ophthalmic artery arising from the posterior surface of the ICA (arrow in a ) and marked in red ( b ).
Fig. 9 Cavernous origin of the ophthalmic artery: MIP TOF lateral view (a), 3D TOF MRA ventral view (b), and 3D rotational DSA lateral view (c) showing the ophthalmic artery (white arrow) arising from the lateral surface of the cavernous segment of the ICA . Note the incidentally discovered Pcom aneurysm (red arrow).
Fig. 10 DSA (a) showing the MMA (red arrow) arising from the ophthalmic artery (white arrow). MIP reconstruction in the CT bone window (b), showing the absence of the foramen spinosum on the left side. Foramen spinosum on the right side is marked for comparison (blue arrow).
Treatment of ischemic stroke in the posterior cerebral artery territory
Cerebral infarctions in the posterior cerebral artery basin are usually secondary and develop against the background of embolism from the underlying segments of the vertebrobasilar system or from the heart cavity. In order to prevent repeated embolisms of the arterial lumen, anticoagulants (heparin) are prescribed. For atherosclerotic occlusion of the posterior cerebral artery, specific treatment is not required. Symptoms of a transient ischemic attack of the brain in the basin of the posterior cerebral artery can be caused by atherothrombotic stenosis of its proximal (underlying) section or one of its penetrating branches (lacunar TIA).
The course of such atherosclerotic lesions of the posterior cerebral artery remains unspecified. Therefore, there are no clear comparative data on the effectiveness of anticoagulants and antiplatelet agents or the prescription of one or another therapy in comparison with its absence. In general, the mildest way to treat ischemia or ischemic stroke in the posterior cerebral artery is to prescribe antiplatelet agents (aspirin, trental).
1.2. Change in the number of vessels
1.2.1. Reducing the number of vessels
ICA agenesis: 0.01% Fig.11 , Fig.47 :
Around day 24 of embryogenesis, the ICA develops from the dorsal aorta and third arch. Subsequently, at approximately the 5th - 6th week, the base of the skull begins to take its shape. Thus, absence of ICA will result in absence of the carotid canal, identification of which is the most practical method in identifying this anomaly in a clinical setting. Typically, patients with ICA agenesis are asymptomatic due to well-developed collateral circulation through the ECA and vertebrobasilar system [2].
Aplasia of the A1 segment: 1-2%. Fig.12 and Fig.15 :
In this situation, both A2 segments are supplied by the existing A1 segment [2].
Azygos ACA: less than 1% Fig. 13 :
Both A1 segments form a common A2 segment, which supplies blood to both hemispheres[2].
Lack of Acom: 5% Fig.14 [2]:
Typically, the absence of the anterior communicating artery (Acom) is not easy to detect on time-of-flight MR angiography because the artery may be present but the flow signal is too weak to be visualized.
Lack of Pcom: 0.6% Fig.15 :
The posterior communicating artery (Pcom) is usually smaller than the P1 segment. Complete absence is rare [2].
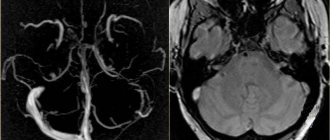
Artery of Percheron: 4-11.5% Fig. 16 :
The thalamo-mesencephalic arterial supply can be divided into 3 types: type 1 is the most common, with perforating arteries on both sides arising from the P1 segments; type 2 , known as the artery of Percheron, arising from one of the P1 segments, supplying both sides; type 3 is an arch that connects both P1 segments and from which the perforating arteries arise [5].
1.2.2. Increase in the number of vessels
Incremental MCA: 2.7% Fig. 17 :
Literary definitions of accessory middle cerebral artery (MCA) and MCA duplication are quite dichotomous. In this paper, we use the definition of Teal et al., who limited the term “accessory MCA” to the branch arising from the anterior cerebral artery (ACA) and the term “duplicate MCA” to the artery arising from the distal segment of the ICA [6]. To distinguish an accessory MCA from a duplex one, the dominant vessel must be identified by carefully searching for the MCA bifurcation. Comparison with the contralateral side is also useful to find the level of ICA bifurcation [1].
Duplication: refers to two separate arteries that do not exhibit distal fusion. For example:
- MCA duplication: 0.2-2.9% Fig. 18 : MCA duplication is an artery that arises from the ICA and runs parallel to the main trunk of the MCA. This variant should not be confused with early branching of the MCA, in which a short single M1 segment is present. It should also not be confused with the anterior temporal branch, which often arises from the M1 segment.
- Acom doubling: 18% Fig. 19 [1].
- SCA doubling: 14% Fig. 12 [2].
Trifurcation:
- ACA trifurcation: 2-13% Fig. 20 : trifurcation refers to the presence of three A2 segments and is described by various names such as pericallosal triplex, arteria mediana corporis callosi and persistent primitive median artery of the corpus callosum [2]. Early origin of a frontopolar branch, for example from Acom, may appear as a third A2 segment.
MCA trifurcation: 12% Fig. 21 : The horizontal segment of the MCA is divided into superior and inferior trunks in approximately 78%. In 12% there is an additional (middle) trunk, this situation is called trifurcation, and the presence of more than 3 trunks, for example, quadrifurcation, is observed in approximately 10% Fig. 22 [2].
Fig. 11 Agenesis of the left ICA. TOF MRA (a), no signal from the flow in the left ICA. MIP CTA (c), CCA continues as ECA with no ICA. Bone window CT (b), absence of bony carotid canal on the left side. The normal carotid duct on the right side is marked with a red arrow for comparison.
Fig. 12 3D MRA, absence of A1 segment of ACA, both A2 segments extend from the contralateral side. Note the partial fetal PCA (white arrow), duplication of the superior cerebellar artery (blue arrow), and hypoplastic vertebral artery (red arrow) that terminates as the PICA.
Fig. 13 3D MRA, fusion of both A1 segments to form a single A2 segment (azygos ACA) (arrow).
Fig.14 3D TOF MRA, no Acom.
Fig. 15 3D TOF, absence of Pcom and A1 segment on one side. The significance of this option is that if the ICA is occluded on that side, there will be no possibility of collateralization through the circle of Willis. Incidental finding: aneurysm of the terminal portion of the contralateral ICA (arrow).
Fig. 16 MIP (a) and 3D TOF (b), type 2 thalamo-mesencephalic arterial supply (artery of Percheron) with a single arterial trunk (arrow) arising from the P1 segment, the branches of which supply blood to both sides.
Fig. 17 3D TOF, additional MCA (arrow) extending from the A1 segment.
Fig. 18 3D TOF of a duplicated MCA (red arrow) extending from the distal ICA. This artery should not be confused with the anterior temporal branch (white arrow), which is a common finding.
Fig. 19 3D TOF, Acom duplication (white arrows), proximal A2 segment fenestration (red arrow), A1 segment aplasia and complete fetal PCA (blue arrow).
Fig. 20 3D TOF, ACA trifurcation with three A2 segments (arrows), the third branch arises from Acom
Fig.21 3D TOF, MCA trifurcation with additional middle trunk.
Fig.22 3D TOF, MCA quadrifurcation.
With a certain periodicity, morphologists of the world gather at their international congresses. The agenda of plenary meetings always includes a report from the nomenclature committee, which makes additions and changes to the unified international anatomical or histological nomenclature. As a rule, these changes are reflected in national versions of nomenclature and terminology publications, which contain the international code of the morphological term, its Latin and national spelling. It is these publications that serve as scientific and methodological standards in the preparation of modern textbooks, manuals, and scientific publications. But, unfortunately, in the international nomenclature printed publications of the last 25-30 years, due to disagreements in determining priorities, the column of eponyms has disappeared. Continuing our series of publications on eponyms [1, 2], we would like to recall the scientists whose names are immortalized in the names of the arteries of the brain.
Artery Bernasconi-Cassinari
- artery of the tentorium of the cerebellum, a branch of the cavernous part of the internal carotid artery, supplying blood to the corresponding derivative of the dura mater. In the English-language literature there are other names - medial or marginal tentorial artery. It was described in 1957 by V. Bernasconi and V. Cassinari based on the results of angiography in a patient with meningioma of the tentorium cerebellum [3, 4].
Italian neuroradiologist V. Bernasconi was educated at the University of Milan. He was professor of radiology (1960) and neuroradiology (1961) in Rome, and headed the neuroradiology clinic in Cagliari (1971-1972) and Milan (1972-1991). One of the founders of the Italian Association of Neuroradiology and the European Neuroradiological Society. The Italian neurosurgeon V. Cassinari (1929-2014) practiced in Bergamo since 1964, where he created his own neurosurgical school. For some time he headed the Italian neurosurgical community of skull base researchers, then became its honorary member. In 1969, together with K. Pagni, he published the monograph “Pain of central origin: a neurosurgical review,” which was then republished.
Circle of Willis.
This anatomical formation is named after the English physician and anatomist T. Willis.
However, he was not the first researcher to pay attention to the anatomical structures that make up this circle. “Father of Anatomy” Herophilus from Chalcedony at the turn of the 4th-3rd centuries. BC. described on the basis of the brain a “wonderful network (of blood vessels).” Mention of this “wonderful network” is also found in the works of C. Galen (131-201 AD), who wrote: “almost under the entire surface of the base of the brain lies this network ... it looks as if they took fishermen’s nets and laid them one on top of the other... its layers are connected to each other, and it is impossible to separate any of them from the rest...” K. Galen believed that the function of the described network is to slow down the blood flow. In the works of Avicenna (980-1037), the description of the “network” is not very different from the dogmatic views of C. Galen: “The network is located under the base of the brain near the main bone, between the dura mater and the bone... the animal spirit needs this network in order to be distributed everywhere, in particular, using the cerebral arteries for this purpose...” Perhaps the first person who decided to publicly express disagreement with this vision of the problem was the Bolognese anatomist. Berengario da Carpi (1460-1530), who rejected the existence of the “network”: “... I examined more than a hundred human heads, paying special attention to the search for this network, and therefore I am well versed in this issue... I have never found any networks... many times I inserted a thin stylus into the said branches located between the ascending parts of the ophthalmic nerves... I found that the stylus penetrated without hindrance directly into the arteries located at the base of the os basilaris
... if the said arteries were divided into thin branches, as Galen says, the stylus could not go all the way to the bottom of the bone... so I believe that Galen invented the miraculous network and never actually saw it... I also believe that other learned men believed in the existence of the miraculous network, rather under the influence of Galen's opinion than because the facts seen..." The famous A. Vesalius in his fundamental book “De Humani Corporis Fabrica” (1543) wrote: “... I myself cannot fully imagine the magnitude of my own stupidity and my too great faith in the works of Galen and other anatomists, yes, I am the same , who did so much out of his love for Galen that he never began a public examination of a man's head without also taking the head of a sheep or an ox, in order to confirm the existence of something that I would never have found in a man's head, and to impress his spectators ... and yet the carotid arteries are hardly capable of forming a reticular plexus in the form in which Galen believed it...”
Anatomists of the post-Vesalian era in their works repeatedly left a description of the “arterial circle” at the base of the brain. G. Fallopio, a student of Vesalius, in 1561 mentioned the existence of an arterial circle at the base of the brain, described the union of two vertebral arteries with their subsequent division, and also noted the presence of a connection between the anterior branches (which correspond to the anterior communicating arteries), but at the same time considered the posterior communicating arteries are only vessels indirectly connected to the carotid arteries. In 1627, D. Casserio depicted an arterial circle in which the posterior communicating artery was present on only one side. In 1658, 6 years before the publication of T. Willis’s book “Cerebrianatome,” the arterial ring of the base of the brain and its anastomotic function were described by Dr. I.Ya. Wepfer. The description was very anatomically accurate, and T. Willis in his writings recognized the significance of this work of his predecessor.
The merit of T. Willis himself lies in the fact that, following in the footsteps of his predecessors, he was able not only to generalize their anatomical observations, but also to substantiate the existence of just such a structure of cerebral vessels.
T. Willis (lat. Willisius) (1621-1675) graduated from the Faculty of Medicine at Oxford University. From 1660 he headed the department of natural philosophy there. In 1667 he moved to London, where he was engaged in private medical practice and scientific research. His monograph “Cerebrianatome” (1664) remained the most significant work on neuroanatomy for 200 years. His description of the structure of the brain, spinal cord, and sympathetic nervous system was far superior to that of his predecessors. He created and presented the first theory of the localization of mental functions, introduced the concept of “neurology,” proposed an improved classification of cranial nerves, and proposed a number of well-known terms (fence, striatum, cerebellar peduncles, pyramids, etc.). T. Willis proved the role of sympathetic nerves in the innervation of the heart and blood vessels, for the first time he described the optic nerve, accessory nerve, internal capsule, anterior commissure, terminal strip, inferior olive, fence; made an attempt to study the structure of the spinal cord and its blood supply [5]. Not only the accessory and optic nerves mentioned above are named in his honor, but also the celiac plexus, the pyloric cave, as well as the phenomenon of paracusia (he noted in this disorder better speech perception in noise than in silence, with otosclerosis).
As a clinician, he differentiated diabetes mellitus and diabetes insipidus (by the taste of urine). He was also the first to attempt a materialistic explanation of the causes and mechanism of oligophrenia, pointing out the need for treatment and special education for children suffering from this pathology [6].
Recurrent artery of Heubner
(long central artery, medial striatal artery) is a branch of the anterior cerebral artery that supplies the head of the caudate nucleus, the anterior limb of the internal capsule and the anterior part of the lentiform nucleus. The artery was first described in 1872 by the German researcher O. Geibner [7]. It was named “Heubner’s artery” in the work of the Massachusetts physician Aitken in 1909, and it began to be called “recurrent” in 1920 after the work of the English anatomist J. Shellshire.
O. Geibner (1843-1926) received his medical education at the University of Leipzig. In 1868 he defended his dissertation on the study of cholera, 5 years later he received the position of professor of internal medicine, and in 1876 he was appointed director of the clinic in Leipzig. Despite his discovery in anatomy, O. Geibner is better known as a figure in children's healthcare - he is considered the “father” of German pediatrics. Thanks to his efforts in 1891 and 1903. The first children's clinics in Germany were opened (in Leipzig and Berlin, respectively), his textbook on pediatrics, written based on his own observations, was republished three times. He was the first professor of pediatrics in Germany, and for 5 years he headed the German Society of Pediatrics, which in 1913 established the Geubner Prize, awarded for contributions to pediatrics. His scientific achievements include the first description of syphilitic endarteritis of the vessels of the base of the brain and celiac enteropathy, the first use of antitoxin for the treatment of diphtheria patients, and the introduction of electrocardiography into pediatric practice. Together with M. Rubner, he described the features of energy metabolism in newborns and, in particular, in premature infants, and established nutritional needs depending on body weight.
Artery of Davidov-Schachter
- the meningeal branch of the posterior cerebral artery, branches in the medial part of the tentorium cerebellum and the posterior region of the falx cerebri. This artery was described by Paul and Gertrud Wollschläger, members of the Department of Neuroradiology at the University of Missouri Medical Center, in 1965, who proposed naming it in honor of their teachers, Davidov and Schechter [8].
Zakharchenko Circle
- a diamond-shaped bulbar arterial ring formed by two vertebral arteries and the anterior spinal arteries extending from them. It is located on the ventral surface of the medulla oblongata and provides its blood supply.
This intersystem anastomosis is named after the Soviet neurologist M.A. Zakharchenko (1879-1953). After graduating from the Faculty of Medicine of the Imperial Moscow University in 1904, he was a practicing physician for 15 years. In 1919 he organized and until 1939 headed the department of nervous diseases at the Turkestan State University, after which he served as a consultant to a group of resorts in the North Caucasus. In 1930 he published one of the first textbooks on nervous diseases in the Soviet Union. M.A. Zakharchenko became famous for his studies of vascular pathology of the brain stem, traumatic injuries and infectious diseases of the nervous system [9].
Artery of Percheron
is a variant of the branching of the posterior cerebral artery. It arises in a single trunk from the initial segment of the left posterior cerebral artery and branches in the paramedian region of the visual thalamus and the rostral part of the midbrain. According to R. Lopez-Serna et al. [10], this artery occurs in 33% of cases. The artery is named after the French scientist J. Percheron (1930-2011), who reported the results of his anatomical studies on the blood supply to the thalamus in 1970 in Munich at a joint meeting of the German and French neurological societies. A printed version of his report appeared three years later in the Zeitschrift für Neurologie.
J. Percheron spent most of his professional career as a researcher at the French Institute of Health and Medical Research, studying the basal ganglia of the brain. In 1983, together with D. McKenzie, he founded the International Society for the Study of the Basal Ganglia (IBAGS), of which he was elected president in 1989. The main of his studies was the fundamental work “Basal ganglia IV - new ideas and data” published in 1992 on structure and function" [11].
Artery Silvia
- middle cerebral artery, branch of the internal carotid artery.
With its superficial branches it supplies blood to most of the cortex of the superolateral surface of the hemispheres, and with its deep branches it supplies the basal ganglia and the internal capsule. This artery was named “Sylvian” by the French anatomist F. Vic-d’Azir in 1786 [12] in honor of the Dutch anatomist F. Silvius (1614-1672), who was the first to describe the sulcus lateralis
(“Sylvian” groove), in which the middle cerebral artery. The cerebral aqueduct and a number of other anatomical structures are also named after this anatomist. F. Silvius should not be confused with the French anatomist J. Silvius (1478-1555), the teacher of A. Vesalius, whose name is immortalized in the eponym “Sylvian ossicle” (lenticular process of the incus).
After studying at the universities of Leiden, Wittenberg, Jena and Basel in 1637, Silvius received his doctorate and then, after a short practice in his native Hanau (Germany), moved to Leiden, where he gave private lessons in anatomy. Subsequently he moved to Amsterdam, where he gained fame as a good doctor and in 1658 became a professor of medicine in Leiden [13].
F. Silvius left a mark on anatomy thanks to his work on the study of the structure of the brain, as well as the fact that he was the first among Dutch scientists to defend and promote the teaching of W. Harvey on blood circulation. Another merit of F. Silvius is the first description (1679) of the pathomorphological picture of pulmonary tuberculosis and the dynamics of its changes. F. Silvius's teaching activity brought him pan-European fame and attracted students from many countries, making Leiden one of the main centers of medical education in Europe. Among his students were the later famous scientists R. de Graaf, J. Swammerdam, T. Bartolin, N. Stenon. Based on the views of Paracelsus and Van Helmont, F. Silvius created the iatrochemical direction in medicine, whose representatives associated the development of diseases with changes in chemical processes in the body [12]. His activities contributed to the transition of medicine from mystical concepts to the rational application of the universal laws of physics and chemistry.
Arteries of Charcot
- the arteries of the lenticular nucleus and striatum are one of the deep branches of the middle cerebral artery, supplying blood to part of the posterior limb of the internal capsule, part of the caudate nucleus, thalamus, putamen, and the outer part of the globus pallidus.
Zh.M. himself Charcot called them “ arteria gemorragica ”
believing that they were the most common source of intracerebral hemorrhages.
The “father” of modern neurology J.M. Charcot (1825-1893), having graduated from the University of Paris in 1848, combined private practice with teaching at the Faculty of Medicine. In 1860 he was confirmed as a professor of neuropathology, and in 1872 of pathological anatomy. From 1862, he headed a department at the Salpêtrière Hospital (Paris), and in 1882, on its basis, he created the first specialized clinic for nervous diseases in Europe, which became a mecca for European neurologists, which he led until his death [14].
Among the merits of Zh.M. Charcot in the early stages of his work included one of the first descriptions of rheumatoid polyarthritis and the first - intermittent claudication and rheumatoid pericarditis, as well as structures later called Charcot-Leyden crystals [15]. He described the symptoms and pathomorphological manifestations of the vast majority of diseases of the nervous system known at that time. The result of his development of the clinical and anatomical direction in neurology was the monograph “On the Localization of Brain Diseases” (1879) - the first work in history on the topical diagnosis of diseases of the nervous system. He also established the psychogenic nature of hysteria, proved the possibility of its development in men and began to widely use hypnosis to treat this disorder, identifying multiple sclerosis and amyotrophic lateral sclerosis as separate diseases. In 1880 J.M. Charcot founded the journal Archives of Neurology. Among his students are world-famous doctors Z. Freud, J. Babinsky, Zh.Zh. Dejerine, P. Marie, J. Wagner-Jauregg, P. Janet and others. For his services, Charcot was elected a full and honorary member of 55 scientific societies and educational organizations.
Thus, eponymous terms remind us of the doctors who considered this problem and demonstrate the continuity of scientific knowledge.
There is no conflict of interest.
1.3. Change in morphology
1.3.1. Hypoplasia
ICA hypoplasia: 0.079% Fig. 23 :
In contrast to agenesis, the thin vessel is identifiable. Again, skull base tomography is useful in visualizing the bony carotid canal, which is thinner than normal in hypoplasia [2].
Hypoplasia A1: 10% Fig.24 :
Asymmetry of A1 segments is observed in 80% of cases. Hypoplasia is defined when the vessel diameter is less than 1.5 mm [2].
Hypoplasia A2 (bihemispheric ACA): 7% Fig. 24 :
One of the A2 segments is hypoplastic. In this variant, the blood supply to the ipsilateral hemisphere occurs mainly from the contralateral (dominant) A2 segment [2].
Hypoplasia Pcom: 34% Fig.25 :
but complete absence is a rare finding [2].
Hypoplasia of the vertebral artery:
50% on the right side (left dominant), 25% on the left side (right dominant), 25% codominant. In approximately 0.2%, the vertebral artery ends in the PICA Fig. 12 and Fig. 26 [7] [2]
1.3.2. Hyperplasia
Hyperplasia of the anterior choroidal artery: 2.3% Fig. 27 :
The anterior choriodal artery arises from the posterior surface of the terminal segment of the ICA, distal to the origin of the Pcom. This is usually a small branch. If it is enlarged (hyperplastic), then it supplies blood to part of the territory of the posterior cerebral artery (occipitotemporal branch) [1, 2].
1.3.3. Early bifurcation (early division):
Early MCA bifurcation: This is a common finding Fig.28 :
The horizontal segment of the MCA is usually 12 mm long, but may be shorter, with early branching (bi- or trifurcation) [1].
1.3.4. Fenestration: 0.7% including all intracranial vessels Fig. 6 , Fig. 19 and Fig. 29 . Fenestration is the division of the lumen of an artery into two separate channels. Each canal has its own endothelium and muscle layer and can separate the adventitia. These two canals merge distally. Fenestration is most often observed in the posterior circulation [1, 2].
- Fenestration A1: 0-4% [1]
- Fenestration A2: 2% Fig. 19 [1]
- Acom fenestration: 12-21% [1]
- Fenestration of the vertebral artery Fig. 29 : 0.3-2% [1].
Fenestration of the basilar artery Fig. 29 : 0.12-1.33%: the basilar artery is formed by the fusion of two longitudinal neural arteries. Incomplete fusion results in segmental fenestration, which is usually present in the proximal segment of the basilar artery [2].
Fig. 23 CTA (a), hypoplastic ICA (arrows). CT bone window (b) asymmetrical bony carotid canal
Fig. 24 3D TOF, hypoplasia of the A2 segment (white arrow) and the contralateral dominant A2 segment “bihemispheric ACA”. Note hypoplasia of the A1 segment (red arrow).
Fig. 25 MIP CTA, right Pcom hypoplasia (arrow). Note the pathological occlusion of the contralateral ICA.
Fig. 26 3D TOF, hypoplasia of the vertebral artery (white arrow) that ends as a PICA (green arrow).
Fig. 27 3D TOF, hyperplasia of the anterior choroidal artery (white arrow). The contralateral anterior choroidal artery is of normal caliber (green arrow). Red arrow points to fetal PCA, blue arrow to Pcom.
Fig. 28 3D MRA, early bifurcation with short prebifurcation segment M1 (arrow).
Fig. 29 Fenestration of the A1 segment (a), Acom (b), M1 segment (c), V4 segment (d) and the proximal part of the basilar artery (e).
Diagnosis and laboratory examination of ischemic stroke in the posterior cerebral artery territory
Infarction in the peripheral territory of the posterior cerebral artery can be easily diagnosed using magnetic resonance imaging (MRI) and computed tomography (). Meanwhile, the data are not sufficiently reliable for infarctions in the central zone of the blood supply of the posterior cerebral artery, especially those developing secondary to occlusive lesions of the penetrating branches of the posterior cerebral artery. Magnetic resonance imaging (MRI) of the brain can detect infarcts of this location with a diameter of more than 0.5 cm.
Angiography remains the only method that convincingly demonstrates atherosclerotic changes or embolic lesions of the posterior cerebral artery. The latest type of modern spiral computed tomography angiography (SCT angiography) with intravenous contrast makes it possible to identify occlusive lesions of small penetrating branches of the posterior cerebral artery.
MRI of the brain of a patient with intracerebral hemorrhage in the thalamus (indicated by arrows).
Thus, diagnosis of ischemic stroke in the posterior cerebral artery basin is based mainly on clinical data confirmed by the results of magnetic resonance imaging (MRI) and computed tomography angiography (CT angiography) with intravenous contrast.
1.4. Changing the course
1.4.1. Aberrant lateral pharyngeal ICA, tortuous ICA, and kissing carotid arteries:
During embryonic development, the ICA is thought to begin to unwind as the dorsal aortic root descends into the thorax, providing a direct pathway for the ICA. Failure in unwinding results in tortuosity of the ICA, which runs close to the midline of the posterior pharyngeal wall, called the aberrant lateral pharyngeal artery [6].
This morphology is more commonly seen in older patients or those with hypertension, but should not be confused with the fetal variant, although both have the same meaning (see below). The incidence of aberrant lateral pharyngeal ICA is approximately 5%, but the exact prevalence of the anomaly is unknown as it cannot be differentiated morphologically from tortuosity. Studies conducted by Ekici et al. showed that the least affected age group with ICA tortuosity was the younger age group [8].
The term "kissing carotid arteries" describes the elongated carotid arteries that meet at the midline; can be observed retropharyngeal or intrasphenoidal / intrasellar Fig. 30 [2].
1.4.2. Persistent primitive olfactory artery: 0,14%
The ACA is derived from the primitive olfactory artery, which regresses to form the recurrent artery of Heubner. Violation of regression leads to preservation of the primitive olfactory artery. This artery has an extreme anterior-inferior course in the A1 segment, which moves along the olfactory tract before the posterosuperior transition to the A2 segment, forming a hairpin-shaped configuration [9].
1.4.3. Persistent embryological anastomosis
+ persistent carotid-vertebrobasilar anastomosis:
During embryonic development, the anterior circulation supplies the hindbrain through several anastomoses, since the posterior circulation is not yet sufficiently developed. After the development of the vertebral arteries, these anastomoses regress. Impaired regression results in abnormal communication between the anterior and posterior circulation in the postnatal period. The most common form of these anastomoses is the fetal type PCA (see variants of origin/origin of vessels). Recognizing the course of these abnormal vessels, as well as the level of entry into the skull, is critical for their differentiation Table 1
- persistent trigeminal artery (PTA): 0.1-0.2% Fig. 31 Fig. 48 : PTA originates from the cavernous segment of the ICA and communicates with the basilar artery. Proximal to the level of the anastomosis, the basilar artery is usually hypoplastic. On the angiogram, when viewed from the side, it has a characteristic “Trident of Neptune” or Tau sign configuration, reminiscent of the Greek letter “Tau” [1] [2]. There are two different classifications Table 2 Fig. 32 and Fig. 33 [10].
- PTA variants (Saltzman III): 0.18-0.76%: Arteries that supply the posterior fossa, arising from the precavernous segment of the ICA and not communicating with the basilar artery [1].
- Persistent auricular artery (otic artery): the rarest carotid-vertebrobasilar anastomosis. The existence of the auricular artery is controversial because it has not been identified in lower animals. It passes from the petrosal segment of the ICA to the basilar system through the internal auditory canal [2].
- Persistent primitive hypoglossal artery (PPHA): 0.03-0.26% Fig. 34 Fig. 49 : This artery runs from the cervical segment of the ICA to the basilar artery through the hypoglossal canal. The vertebral artery is hypoplastic. CT scan of the skull base shows an enlarged bony hypoglossal canal [1, 2].
- Proatlantal intersegmental artery: very rare. Connects the cervical segment of the ICA or external carotid artery (ECA) to the vertebrobasilar system. The artery enters the base of the skull through the foramen magnum, which allows it to be differentiated from the hypoglossal artery. There are two types:
- Type I: joins the vertebral artery above the atlas.
- Type II: enters the vertebral artery through the atlas [1, 2].
+ persistent internal-external carotid anastomosis
- Aberrant intratympanic ICA: very rare. This variant is an anastomosis between the ICA and the ECA, as it is believed to arise from agenesis of the cervical segment of the ICA and the development of an anastomosis between the horizontal (petrosal) segment of the ICA and the enlarged inferior tympanic artery, which is a branch of the ECA. The ICA (or rather the enlarged inferior tympanic artery) in this case has a smaller diameter than the usual ICA, with the absence of the ascending part of the carotid canal as it enters the base of the skull, posterior and parallel to the jugular bulb, which resembles a mass in the hypotympanum; there is also no bone plate between the carotid canal and the tympanic cavity [1].
- Persistent stapedial artery: 0.48%. This anomaly occurs due to the persistence of the anastomosis through the stapedial artery, which is usually present during development between the ECA and ICA. The artery arises from the petrosal segment of the ICA, passes through the obturator foramen, and ends as the MCA in the epidural space of the middle cranial fossa. A CT scan of the skull base may show a small canal near the carotid canal. Foramen spinosum, which contains MMA, will be absent. a persistent stapedial artery may be associated with an aberrant ICA [1, 2].
Table 3 shows the frequency of variants discussed, however, there are other rare variants that cannot be included in a single document. Finally, having a fully developed Circle of Willis can be considered an option since it is present in less than 50% of the population [2]
*NB: Frequency varies between authors depending on the type of study performed (CT, MRI, surgical or post-mortem). Frequency may also vary depending on geographic distribution; Published data may not always be applicable to other populations.
Table 1: Types of persistent carotid-vertebrobasilar anastomoses
Table 3: occurrence of anatomical variants
Fig. 30 Coronal MIP CTA of a patient with a history of hypertension shows elongated carotid arteries reaching the midline (“kissing” carotid arteries).
Fig. 31 3D TOF, lateral view (a) and dorsal view (b), persistent primitive trigeminal artery (red arrow) arising from the cavernous segment of the ICA and communicating with the basilar artery, which is hypoplastic to the level of the anastomosis (white arrow). In lateral view, the anomalous artery with ICA resembles Neptune's trident and the Greek letter "tau".
Fig. 32 MIP CTA of persistent primitive trigeminal artery (red arrow) in two different cases. According to Salas there are 2 types: medial sphenoidal or intrasellar (a), which extends into the sella turcica and perforates the dura mater or dorsum sella (green arrow), as in this case, and lateral petrosal or parasellar (b), in which the vessel goes with the sensory roots of the trigeminal nerve, on the side of the sella turcica.
Fig. 33 3D TOF showing two different cases of persistent primitive trigeminal artery (red arrow). Classification according to Saltzman: Type I (a), in which the PCA supplies the superior part of the basilar artery, including the posterior territory, and type II (b) with the fetal PCA (white arrow).
Fig. 34 3D CE MRA oblique (a) and posterior (b) views showing the persistent primitive hypoglossal artery (red arrow) which arises from the cervical segment of the ICA (green arrow) and continues as the vertebrobasilar artery (blue arrow). ECA is marked with a white arrow.
Fetal Doppler: weekly norm and prognosis for deviations
In order for the results to be deciphered correctly and all deviations to be identified, it is necessary to compare the data obtained with standard values, taking into account the gestational age.
Indicators of the norm of the uterine artery resistance index
| Gestational period (weeks) | Average IR of the uterine arteries | Possible range of fluctuations |
| 20 | 0,52 | 0,37 – 0,7 |
| 21 | 0,51 | 0,36 – 0,69 |
| 22 | 0,5 | 0,36 – 0,68 |
| 23 | 0,5 | 0,36 – 0,68 |
| 24 | 0,5 | 0,35 – 0,67 |
| 25 | 0,49 | 0,35 – 0,66 |
| 26 | 0,49 | 0,35 – 0,65 |
| 27 | 0,48 | 0,34 – 0,64 |
| 28 | 0,48 | 0,34 – 0,64 |
| 29 | 0,47 | 0,34 – 0,63 |
| 30 | 0,46 | 0,34 – 0,62 |
| 31 | 0,46 | 0,34 – 0,61 |
| 32 | 0,45 | 0,34 – 0,61 |
| 33 | 0,45 | 0,34 – 0,59 |
| 34 | 0,45 | 0,34 – 0,59 |
| 35 | 0,45 | 0,33 – 0,58 |
| 36 | 0,44 | 0,33 – 0,58 |
| 37 | 0,44 | 0,33 – 0,57 |
| 38 | 0,44 | 0,33 – 0,57 |
| 39 | 0,43 | 0,33 – 0,57 |
| 40 | 0,43 | 0,32 – 0,57 |
| 41 | 0,43 | 0,32 – 0,56 |
Standard indicators of the pulsatility index of the uterine arteries
| Gestational period (weeks) | Average PI of the uterine arteries | Possible range of fluctuations |
| 20 | 1,54 | 1,04 – 2,03 |
| 21 | 1,47 | 0,98 – 1,96 |
| 22 | 1,41 | 0,92 – 1,9 |
| 23 | 1,35 | 0,86 – 1,85 |
| 24 | 1,3 | 0,81 – 1,79 |
| 25 | 1,25 | 0,76 – 1,74 |
| 26 | 1,2 | 0,71 – 1,69 |
| 27 | 1,16 | 0,67 – 1,65 |
| 28 | 1,12 | 0,63 – 1,61 |
| 29 | 1,08 | 0,59 – 1,57 |
| 30 | 1,05 | 0,56 – 1,54 |
| 31 | 1,02 | 0,53 – 1,51 |
| 32 | 0,99 | 0,5 – 1,48 |
| 33 | 0,97 | 0,48 – 1,46 |
| 34 | 0,95 | 0,46 – 1,44 |
| 35 | 0,94 | 0,44 – 1,43 |
| 36 | 0,92 | 0,43 – 1,42 |
| 37 | 0,92 | 0,42 – 1,41 |
| 38 | 0,91 | 0,42 – 1,4 |
| 39 | 0,91 | 0,42 – 1,4 |
| 40 | 0,91 | 0,42 – 1,4 |
| /41 | 0,92 | 0,42 – 1,41 |
Indicators of the right and left uterine artery may be different. The main thing is that both indicators do not go beyond the norm. If both indicators are not normal, this indicates a violation of the uteroplacental circulation. If one indicator is for asymmetry of uteroplacental blood flow
It is important to note that at 18-21 weeks, deviations in indicators may be observed due to the incomplete adaptive physiological process of cytotrophoblast invasion. In this case, Doppler testing of the fetus should be repeated after 2-3 weeks.
Standard indicators of the systole-diastolic ratio in the fallopian tubes
| Gestational period (weeks) | SDO norm |
| 20 – 24 | up to 2.5 |
| 25 – 27 | up to 2.4 |
| 28 – 33 | up to 2.3 |
| 34 – 41 | up to 2.3 |
Normal Doppler measurement: umbilical cord arteries
Standard values of the umbilical cord artery resistance index:
| Gestational period (weeks) | Average index of IR of the umbilical cord arteries | Possible range of fluctuations |
| 20 | 0,74 | 0,63 – 0,84 |
| 21 | 0,73 | 0,62 – 0,83 |
| 22 | 0,72 | 0,61 – 0,82 |
| 23 | 0,71 | 0,6 – 0,82 |
| 24 | 0,7 | 0,59 – 0,81 |
| 25 | 0,69 | 0,58 – 0,8 |
| 26 | 0,68 | 0,58 – 0,79 |
| 27 | 0,67 | 0,57 – 0,79 |
| 28 | 0,66 | 0,56 – 0,78 |
| 29 | 0,65 | 0,55 – 0,78 |
| 30 | 0,64 | 0,54 – 0,77 |
| 31 | 0,63 | 0,53 – 0,76 |
| 32 | 0,62 | 0,52 – 0,75 |
| 33 | 0,61 | 0,51 – 0,74 |
| 34 | 0,6 | 0,49 – 0,73 |
| 35 | 0,59 | 0,48 – 0,72 |
| 36 | 0,58 | 0,46 – 0,71 |
| 37 | 0,57 | 0,44 – 0,7 |
| 38 | 0,56 | 0,43 – 0,69 |
| 39 | 0,55 | 0,42 – 0,68 |
| 40 | 0,54 | 0,41 – 0,67 |
| 41 | 0,53 | 0,4 – 0,66 |
Standard values of the pulsatility index of the umbilical cord arteries:
| Gestational period (weeks) | Average PI of the umbilical cord arteries | Possible range of fluctuations |
| 18 | 1,72 | 1,53 – 1,9 |
| 19 | 1,62 | 1,45 – 1,78 |
| 20 | 1,45 | 1,25 – 1,65 |
| 21 | 1,35 | 1,18 – 1,51 |
| 22 | 1,35 | 1,17 – 1,52 |
| 23 | 1,25 | 1,09 – 1,41 |
| 24 | 1,12 | 0,96 – 1,27 |
| 25 | 1,15 | 0,98 – 1,33 |
| 26 | 1,01 | 0,86 – 1,16 |
| 27 | 1,01 | 0,86 – 1,16 |
| 28 | 1,05 | 0,87 – 1,23 |
| 29 | 1,03 | 0,88 – 1,17 |
| 30 | 0,95 | 0,76 – 1,13 |
| 31 | 0,85 | 0,71 – 0,99 |
| 32 | 0,84 | 0,67 – 1,1 |
| 33 | 0,84 | 0,59 – 0,93 |
| 34 | 0,83 | 0,58 – 0,99 |
| 35 – 37 | 0,81 | 0,57 – 1,05 |
| 38 – 41 | 0,74 | 0,37 – 1,08 |
Obtaining zero and reverse values of diastolic blood flow is considered a pathology. This indicates a critical condition of the fetus, the death of which will occur in 2-3 days. In this case, a caesarean section is immediately prescribed (if the gestational age is more than 28 weeks) to save the baby's life.
Standard values for the systole-diastolic ratio of the umbilical cord arteries:
| Gestational period (weeks) | SDO norm |
| 20 – 24 | up to 4.4 |
| 25 – 27 | up to 3.8 |
| 28 – 33 | up to 3.2 |
| 34 – 41 | up to 2.9 |
Impaired blood flow in the umbilical cord entails a delay in the development of the child.
Doppler ultrasound norms: middle cerebral artery of the fetus
| Gestational period (weeks) | Average PI in the middle cerebral artery | Possible range of fluctuations |
| 20 | 1,83 | 1,36 – 2,31 |
| 21 | 1,87 | 1,4 – 2,34 |
| 22 | 1,91 | 1,44 – 2,37 |
| 23 | 1,93 | 1,47 – 2,4 |
| 24 | 1,96 | 1,49 – 2,42 |
| 25 | 1,97 | 1,51 – 2,44 |
| 26 | 1,98 | 1,52 – 2,45 |
| 27 | 1,99 | 1,53 – 2,45 |
| 28 | 1,99 | 1,53 – 2,46 |
| 29 | 1,99 | 1,53 – 2,45 |
| 30 | 1,98 | 1,52 – 2,44 |
| 31 | 1,97 | 1,51 – 2,43 |
| 32 | 1,95 | 1,49 – 2,41 |
| 33 | 1,93 | 1,46 – 2,39 |
| 34 | 1,9 | 1,43 – 2,36 |
| 35 | 1,86 | 1,4 – 2,32 |
| 36 | 1,82 | 1,36 – 2,28 |
| 37 | 1,78 | 1,32 – 2,24 |
| 38 | 1,73 | 1,27 – 2,19 |
| 39 | 1,67 | 1,21 – 2,14 |
| 40 | 1,61 | 1,15 – 2,08 |
| 41 | 1,55 | 1,08 – 2,01 |
Maximum velocity in the fetal middle cerebral artery:
| Gestational period (weeks) | Average indicator | Possible range of fluctuations |
| 19 | 19,7 | 16,7 – 23 |
| 20 | 21,8 | 18,1 – 26 |
| 21 | 23,9 | 19,5 – 29 |
| 22 | 26 | 20,8 – 32 |
| 23 | 28,2 | 22,2 – 35 |
| 24 | 30,3 | 23,6 – 38,1 |
| 25 | 32,4 | 24,9 – 41,1 |
| 26 | 34,6 | 26,3 – 44,1 |
| 27 | 36,7 | 27,7 – 47,1 |
| 28 | 38,8 | 29 – 50,1 |
| 29 | 40,9 | 30,4 – 53,1 |
| 30 | 43,1 | 31,8 – 56,1 |
| 31 | 45,2 | 33,1 – 59,1 |
| 32 | 47,3 | 34,5 – 62,1 |
| 33 | 49,5 | 35,9 – 65,1 |
| 34 | 51,6 | 37,2 – 68,2 |
| 35 | 53,7 | 38,6 – 71,2 |
| 36 | 55,8 | 40 – 74,2 |
| 37 | 58 | 41,3 – 77,2 |
| 38 | 60,1 | 42,7 – 80,2 |
| 39 | 62,2 | 44,1 – 83,2 |
| 40 | 64,4 | 45,4 – 86,2 |
Standard values for the systolic-diastolic ratio in the middle cerebral artery:
| Gestational period (weeks) | SDO norm |
| 20 – 24 | not less than 2.9 |
| 25 – 27 | not less than 2.7 |
| 28 – 33 | not less than 2.4 |
| 34 – 41 | not less than 2.2 |
Normal fetal Doppler findings: fetal aorta
Disturbances in the blood circulation of the fetal aorta can be detected only after 22-24 weeks of pregnancy.
Standard values of the pulsatility index of the fetal aorta:
| Gestational period (weeks) | Average PI of the fetal aorta | Possible range of fluctuations |
| 20 | 1,79 | 1,49 – 2,16 |
| 21 | 1,79 | 1,49 – 2,16 |
| 22 | 1,79 | 1,49 – 2,17 |
| 23 | 1,8 | 1,49 – 2,18 |
| 24 | 1,8 | 1,49 – 2,19 |
| 25 | 1,81 | 1,49 – 2,2 |
| 26 | 1,81 | 1,49 – 2,21 |
| 27 | 1,82 | 1,5 – 2,22 |
| 28 | 1,83 | 1,5 – 2,24 |
| 29 | 1,82 | 1,51 – 2,25 |
| 30 | 1,81 | 1,51 – 2,26 |
| 31 | 1,81 | 1,52 – 2,28 |
| 32 | 1,8 | 1,53 – 2,29 |
| 33 | 1,8 | 1,53 – 2,31 |
| 34 | 1,79 | 1,54 – 2,32 |
| 35 | 1,79 | 1,55 – 2,34 |
| 36 | 1,79 | 1,55 – 2,35 |
| 37 | 1,92 | 1,56 – 2,36 |
| 38 | 1,93 | 1,57 – 2,38 |
| 39 | 1,94 | 1,57 – 2,39 |
| 40 | 1,94 | 1,57 – 2,4 |
| 41 | 1,95 | 1,58 – 2,41 |
Standard values of the fetal aortic resistance index:
| Gestational period (weeks) | Average IR of the fetal aorta | Possible range of fluctuations |
| 20 – 26 | 0,79 | 0,68 – 0,87 |
| 27 – 34 | 0,79 | 0,67 – 0,87 |
| 35 – 41 | 0,78 | 0,66 – 0,87 |
Standard values for fetal aortic systolic velocity:
| Gestational period (weeks) | Average systolic velocity | Possible range of fluctuations |
| 20 | 26,88 | 12,27 – 44,11 |
| 21 | 28,87 | 14,1 – 46,28 |
| 22 | 30,52 | 15,6 – 48,12 |
| 23 | 31,95 | 16,87 – 49,74 |
| 24 | 33,23 | 18 – 51, 2 |
| 25 | 34,39 | 19 – 52,55 |
| 26 | 35,47 | 19,92 – 53,81 |
| 27 | 36,47 | 20,77 – 55,01 |
| 28 | 37,42 | 21,55 – 56,13 |
| 29 | 38,32 | 22,3 – 57,22 |
| 30 | 39,17 | 23,02 – 58,26 |
| 31 | 40,01 | 23,66 – 59,27 |
| 32 | 40,8 | 24,3 – 60,26 |
| 33 | 41,57 | 24,92 – 61,21 |
| 34 | 42,32 | 25,52 – 62,16 |
| 35 | 43,06 | 26,1 – 63,08 |
| 36 | 43,79 | 26,67 – 64,02 |
| 37 | 44,52 | 27,24 – 64,93 |
| 38 | 45,24 | 27,8 – 65,81 |
| 39 | 45,96 | 28,37 – 66,72 |
| 40 | 46,7 | 28,95 – 67,65 |
| 41 | 47,47 | 29,57 – 68,62 |
Standard values for the systolic-diastolic ratio of the fetal aorta:
| Gestational period (weeks) | SDO norm |
| 20 – 24 | up to 8.4 |
| 25 – 27 | up to 8.2 |
| 28 – 33 | up to 7.9 |
| 34 – 41 | up to 7.4 |
Doppler norms during pregnancy: ductus venosus
The ductus venosus is not assessed using indices. An indicator of pathology is zero or negative blood flow values. Typically, similar values are obtained for fetal malnutrition, congenital heart disease, and nonimmune hydrops.
In the case when the blood circulation in the umbilical cord is in a critical condition, but no blood flow deviations were detected in the venous duct, it is possible to extend gestation until the optimal period for delivery.
Meaning
The list in Fig. 35 shows the values of the anatomical options.
2.1. Recognition of anatomical patterns and the ability to distinguish them from pathological changes:
2.1.1. Knowledge of normal variations is part of the anatomical knowledge that is important for every radiologist and surgeon. Knowledge of normal variants and their proximity to other structures facilitates the understanding and diagnosis of various diseases, such as:
- Trigeminal neuralgia, which can be caused by the presence of a variant of PTA (less commonly PTA), due to the proximity of the vessel to the trigeminal nerve [11].
- Glossopharyngeal neuralgia or hypoglossal nerve palsy, which can be caused by a persistent hypoglossal artery [1].
- Pulsatile tinnitus in cases of persistent stapedial artery [1].
2.1.2. Option against pathology:
- Infundibulum: The Pcom infundibulum should not be confused with an aneurysm Fig. 36 .
- ICA hypoplasia may be confused with dissection or fibromuscular dysplasia, while ICA agenesis may be confused with occlusion. Visualization of the skull base aids differentiation, as the bony carotid canal will be narrow in cases of hypoplasia and absent in cases of agenesis, but will appear normal in other acquired diseases Fig. 11 and Fig. 23 .
- Different patterns of perfusion abnormalities may occur with normal variations that can cause confusion, especially in the context of stroke:
- Asymmetry of CT or MR perfusion in the occipital lobes, in the case of unilateral fetal PCA. The contralateral side may show delayed perfusion because it is supplied by the posterior circulation Fig. 37 .
- Bilateral perfusion delay in the occipital lobes compared with the frontal and parietal lobes may be observed in the absence of bilateral Pcom Fig. 38 .
- Relative hypoperfusion in the PICA territory in cases of vertebral artery hypoplasia. Hypoperfusion may present as prolonged time-to-peak, prolonged main transit time, or decreased cerebral blood flow, but it never affects cerebral blood volume ) Fig.39 [12].
2.2. Hemodynamic effect of normal variants and abnormalities:
2.2.1. Understanding collateral function: The presence of hypoplasia or aplasia of segment(s) in the circle of Willis can affect collateral function when one or more arteries are occluded Fig. 15 .
2.2.2. Explains unclear cases of stroke:
Vascular conditions that cause changes in unexpected vascular territories can be explained by normal variations such as:
- Ischemia in the posterior territory may accompany ICA pathology due to the presence of fetal PCA Fig. 40 .
- Bilateral ischemia and ischemia in certain areas may draw attention to the presence of pathology in one of the options, such as:
- Bilateral anterior infarction in case of thromboembolism of azygos ACA or dominant bihemispheric ACA Fig. 41 .
- Bilateral mesencephalothalamic infarction with Percheron's artery Fig. 42 .
2.3. Association with vascular and nonvascular congenital anomalies and other diseases:
2.3.1. Association with aneurysms: Changes in vascular anatomy may be a sign of lack of vascular maturity and vulnerability to aneurysm formation. In the work of Lazzaro et al., normal variants of the circle of Willis were more common in cases with ruptured aneurysms than in cases of unruptured aneurysms [13]. Based on a review of the literature, the following variants and abnormalities were associated with aneurysms: Table 4 Fig. 43
- Fenestrations: The incidence of aneurysms (IoA) is approximately 7% of all fenestrations. A defect in the media of the fenestrated segment and turbulent flow at both ends of the fenestration can lead to aneurysm formation. Additionally, in the work of Hudák et al., fenestration was a common finding in patients with unexplained subarachnoid hemorrhage due to a weak arterial wall [1] [2] [14]
- ICA agenesis and hypoplasia: IoA 67% [2].
- A1 segment aplasia: IoA14% [15].
- Azygos ACA: IoA 41%. Due to increased flow from both segments of A1 [2].
- Persistent dorsal ophthalmic artery: IoA 45% [4].
- Persistent primitive olfactory artery: In the work of Uchino et al, 2 intracranial artery aneurysms were found in 14 patients with PPOA (IoA about 14%); one of them is in the hairpin bend (7%) [9].
- PTA: IoA 14% [1].
- Persistent hypoglossal artery: IoA 26% [16].
- Proatlantal intersegmental artery: IoA 10% [1, 2]
- Other variants and anomalies associated with aneurysms whose cases have been reported but not available include infraoptic ACA, superior anterior communicating artery, accessory MCA, MCA aplasia, variant PTA, and asymmetry of the circle of Willis [1, 2].
2.3.2. Association with other vascular anomalies and diseases:
- Fenestration of the vertebral artery is associated with arteriovenous malformation in 7% [6].
- PTA is observed in vascular anomalies such as AVM, carotid-cavernous fistula, and Moyamoya disease in 25% of cases [17].
- Proatlantal intersegmental artery: The incidence of cerebrovascular disorders such as AVM, vein of Galen malformation and aortic arch variants is 59% [18].
- Spontaneous vertebral artery dissection was slightly more common in subjects with hypoplastic vertebral artery than in controls (30.4% vs. 17.4%). It was also found that spontaneous vertebral artery dissection occurs more often with hypoplastic vertebral arteries than with dominant vertebral arteries (68% versus 32%) [19].
2.3.3. Association with other congenital anomalies:
- Azygos ACA may be associated with holoprosencephaly and migration abnormalities Fig. 44 [1].
- ICA hypoplasia is associated with anencephaly and basal telangiectasia [2].
- Fenestration of the vertebral artery may be associated with vertebral fusion [6].
2.3.4. Association with other disorders:
- Pituitary dysfunction and acromegaly in intrasellar “kissing” carotid arteries[2].
- It was found that migraine with aura is more common in patients with an open circle of Willis [20].
2.4. Preoperative planning for cranial surgery, head and neck surgery, and neurointerventional procedures:
The description of normal variations is very important for surgeons and interventional radiologists, as some of these variations must be considered to avoid catastrophic consequences during intervention.
2.4.1. The risk of catastrophic hemorrhage exists in the following cases:
- Transsphenoidal pituitary surgery in cases of PTA or intrasellar “kissing” carotid arteries.
- Middle ear surgery in cases of persistent stapedial artery and aberrant intratympanic ICA.
- Pharyngeal surgeries such as otopharyngeal tumor resection, tonsillectomy, adenoidectomy and palatopharyngoplasty in cases of aberrant lateral pharyngeal artery.
2.4.2. Knowledge of normal variations is important in interventional procedures. This knowledge may help to gain vascular access, such as dominant versus hypoplastic vertebral or variant access, or avoid complications during procedures such as tumor embolization through ECA catheterization in cases of MMA arising from the ophthalmic artery, which can lead to blindness . Fig.45 Fig.50
2.4.3. The presence of persistent carotid-basilar anastomoses should be excluded before certain procedures, such as the Wada test: in this case, injection of amytal can lead to loss of consciousness and apnea [2]
Fig.35 Meaning of normal options
Table 4: Variants associated with aneurysms and their occurrence
Fig. 36 3D TOF MRA, funnel-shaped dilatation with origin of Pcom (white arrow), which should not be confused with an aneurysm. Note the small aneurysm at the origin of the contralateral Pcom (red arrow). Also note the trifurcation of the ACA.
Fig. 37 MR perfusion, TTP map (a) shows slow perfusion in the left occipital lobe; no abnormalities were found in other perfusion parameters. 3D TOF (b), showing fetal PCA on the contralateral side (arrow).
Fig. 38 TTP perfusion map (a) shows a symmetrical delay in the occipital lobes, no deviations in other perfusion parameters were noted. 3D TOF shows no Pcom on both sides.
Fig. 39 (same patient as in Fig. 12) with hypoplasia of the right vertebral artery, which ends as the PICA. TTP map shows perfusion delay in the territory of the right PICA. An examination performed 6 weeks later for other reasons (not shown) showed no pathology in this area.
Fig. 40 MRI of a 56-year-old patient complaining of headache shows dissection of the left ICA (red arrows) with intramural hematoma on T2 (a) and T1FS (b). DWI (c) and ADC (d) show subacute infarction in the territory of the left PCA, which is due to the presence of fetal PCA (TOF not shown).
Fig.41 72-year-old patient with hemiplegia, epilepsy and impaired consciousness. DWI (a) and ADC (b) show bilateral infarcts in the ACA territory. DSA (c), shows proximal occlusion of the azygos ACA (arrow).
Fig. 42 Bilateral acute thalamic infarctions on DWI (a). DSA shows occlusion of the P1 segment of the left PCA (b). Minimal recanalization after intra-arterial thrombolysis (c), with mild opacification of the artery of Percheron (arrows) arising from the left PCA.
Fig. 43 Aneurysms (arrows) associated with abnormalities; a) A1 aplasia with Acom aneurysm. b) Azygos ACA with pericallosal aneurysm. c) fenestration of the basilar artery with proximal basilar aneurysm after coiling.
Fig. 44 Axial (a) and sagittal (b) MRI images of a child with holoprosencephaly, anterior cingulate fusion, and abnormal beak and genu corpus callosum. Note the empty flow of the anterior cerebral artery (arrow), which is single (azygos) and displaced anteriorly.
Fig. 45 55-year-old patient involved in an accident. Initial CT scan (a) shows a fracture of the left temporal bone. She later complained of pulsating tinnitus. DSA (c) with flat panel CT angiography (b and d) was performed. Reconstructed images showed the middle meningeal artery (yellow arrows) arising from the ophthalmic artery (blue arrow) and the traumatic AVM (red arrows) draining into the external jugular vein to form an aneurysm (orange arrow). Knowledge of this option is important when planning therapy.
Abbreviations:
- A1: Horizontal segment of ACA
- A2: Vertical segment of ACA
- ACA: Anterior cerebral artery
- Acom: Anterior communicating artery
- AICA: Anterior inferior cerebellar artery
- AVM: Arteriovenous malformation
- BA: Basilar artery
- CBF: Cerebral blood flow
- CBV: Cerebral blood volume
- CoW: Circle of Willis
- DSA: Digital subtraction angiograph
- ECA: External carotid artery
- ICA: Internal carotid artery
- IoA: Incidence of aneurysms
- M1: Sphenoidal segment of MCA
- MCA: Middle cerebral artery
- MMA: Middle meningeal artery
- MTT: Mean transit time
- PCA: Posterior cerebral artery
- Pcom: Posterior communicating artery
- PDOA: Persistent dorsal ophthalmic artery
- PICA: Posterior inferior cerebellar artery
- PPHA: Persistent primitive hypoglossal artery
- PPOA: Persistent primitive olfactory artery
- PTA: Persistent trigeminal artery
- SCA: Superior cerebellar artery
- sVAD: Spontaneous vertebral artery dissection