Neurologist
SEMENOVA
Olga
5 years experience
neurologist, head of the office for diagnostics and treatment of cognitive disorders
Make an appointment
Polyneuropathies (polyneuropathies) are a group of pathologies affecting peripheral nerves. The clinical picture is determined by the form of the disease diagnosed in the patient. Specific signs are muscle weakness and atrophy, decreased tendon reflexes, sensory disturbances, and autonomic disorders. Treatment of polyneuropathy is carried out by a neurologist. The main task of the doctor is to determine the cause of the disease. Therapy is symptomatic.
Causes
The reasons leading to the development of polyneuropathy in patients are varied. Often, damage to nerve cells develops against the background of severe poisoning with methyl alcohol, arsenic or carbon monoxide. Similarly, axonal health can be affected by volatile compounds high in phosphorus.
In some cases, the symptoms of the pathology appear against the background of chronic intoxication of the human body, diphtheria, vitamin deficiency or diabetes mellitus. The disease can develop with uremia, liver cirrhosis, kidney damage, hypothyroidism or cancer. Long-term use by the patient of amiodarone, isoniazid or metronidazole can lead to the manifestation of polyneuropathy.
Disability in chronic polyneuropathy
Natasha
1752 views
November 28, 2020
Good afternoon!Female, 30 years old!I fell ill with polyneuropathy in November 2019, when I noticed a sharp decrease in vision in one eye, in 3 days it dropped from -4 to -10, on the MRI there were foci of gliosis, edema, and a pineal gland cyst. She was treated in a hospital. my vision was restored, I was discharged, after 2 months of treatment I became very ill with the flu and... PNP returned. Treatment again for 2 months, but at home, because covid came, they treated me, and again PNP and so 4 or 5 outbreaks. I came back. to a neurologist again, sent for examination. Cardiologist - postmyocardium, cardiosclerosis and rare extrasystole. Rheumatologist - osteoarthritis, bursitis, trochanteritis and mild osteoporosis, spondyloarthrosis of the populace, scoliosis and osteochondrosis. Ultrasound of the kidneys - cyst of the right kidney. Endocrinologist - b/o , everything is fine. Other existing diseases: chronic tonsillitis, chronic gastritis, chronic cholecystitis, chronic gastrointestinal anemia, lipomatosis, emphysema, cyst and pneumofibrosis in the right lung, hyperplasia with cystic inclusions in the nasopharyngeal tonsils. Operations: 2010 - thoracotomy and middle lobectomy of the middle lobe of the right lung, lymphadenectomy of the lower lymph node, 2012 - apoplexy of the left ovary, 2015 - laparotomy, rupture of the left ovarian cyst. ENMG complete absence of reflexes on both sides of the lower extremities, axonal sensory disturbances. -ty and impaired reflex excitability. Something like this in general. I was fired from work because of this, because I have frequent sick days. I can’t work because I have pain: numbness of the limbs, burning, tingling, weakness in the body , pain in the knees, hips, back... I visited a therapist and asked about registration of disability, because the neurologist said that PNP is now of a chronic form and you can try to register it, so at least there will be money for medications and permanent courses of treatment, the neurologist believes that PSP as a result of a connective tissue disorder, or hereditary, it seems like this. The therapist looked at a couple of pieces of paper: only ENMG and a neurologist’s report and said that they would not give me disability. And he said that he does not believe that I have such severe pain, I do not have pain relief with expensive anticonvulsants funds. She didn’t even ask anything about chronic diseases and didn’t look at all my examinations. The question is, will they give me disability, is it worth trying, do I have the right? My performance is reduced, I don’t know what to do. And yes, treatment this time It doesn’t help, neither from a rheumatologist, nor from a neurologist, it’s just a little easier.
The question is closed
disability
Polyneuropathy
Kinds
Neurologists distinguish two types of polyneuropathies based on pathogenetic signs of damage to peripheral nerves - axonal and demyelinating. The first form of the disease affects the long, cylindrical extensions of nerve cells called axons. The second provokes the loss of myelin in myelinated nerve fibers. Late stages of axonal disorders are accompanied by demyelination. Primary demyelinating polyneuropathies are complicated by secondary damage to the cylindrical processes of nerve cells.
In the process of diagnosing and treating polyneuropathies, doctors may use other classifications of the disease. Based on the clinical picture, neurologists distinguish motor, sensory and autonomic polyneuropathies. In their pure form, these types of pathology are rare: most patients are diagnosed with damage to two or three types of nerve fibers (motor-sensory, sensory-vegetative, etc.).
Studying the etiology of the disease allows neurologists to identify hereditary, autoimmune, metabolic, nutritional, toxic and infectious-toxic polyneuropathies.
List of required examinations for ITU
Which disability groups are considered workers?
As with all other diseases, obtaining disability due to polyneuropathy requires passing a certain minimum of examinations, the forms of which must subsequently be submitted to the ITU.
This list includes:
- General (clinical) blood and urine tests.
- Diagnosis of cerebrospinal fluid (necessary for patients suffering from infectious polyneuropathies, as well as autoimmune ones).
- Ophthalmological, somatic types of diagnostics, determined by the origin of the disease.
- EMG (electromyography), ENMG (electroneuromyography) (procedures are recommended in dynamics).
- Virological, bacteriological studies, based on the peculiarities of the occurrence of polyneuropathy.
- RVG (rheovasography - study of blood supply to the extremities), thermal imaging.
- Biochemical diagnostics of blood and urine (necessary for toxic, somatic polyneuropathies, as well as for the porphyritic form of the disease).
In addition, in the opinion of the attending physician, additional types of research may be required, which is determined by the peculiarities of the etiology of polyneuropathy, the nature, and the severity of its course.
Symptoms of pathology
During the examination of the patient, the neurologist identifies signs of damage to motor, sensory and autonomic fibers. The degree of involvement of these structures in the pathological process determines the clinical picture of the disease. Thus, when occupational polyneuropathy is detected in men and women, neurologists record sensory-vegetative symptoms (which are associated with regular fixation of muscles in a state of static tension).
The predominance of motor signs of pathology becomes the cause of the development of paresis of the upper and lower extremities. In the absence of treatment, the patient's individual muscle groups atrophy, including the respiratory ones (against the background of Guillain-Barré syndrome). Later, tendon reflexes decrease, and the person has difficulty performing basic tasks such as fastening buttons or tying shoelaces.
Sensory symptoms develop symmetrically, affecting the feet and hands of patients in the early stages. Sensitivity disorder manifests itself in different ways. Patients may experience tingling, burning, numbness, and pain. In the later stages of the disease, adults and children experience disturbances in the pain and temperature sensitivity of the skin.
Autonomic disorders are accompanied by dry skin and dysregulation of vascular tone. Over time, patients develop signs of tachycardia and unstable functioning of the gastrointestinal tract. Men may experience erectile dysfunction.
Indications for passing the ITU
The attending physician refers the patient for MSA if the following health deviations are observed, which, in his opinion, can become an obstacle to the performance of assigned work duties. These include:
- Paresis and paralysis of the arms and legs, persistent pain, severe trophic and autonomic disorders, sensitive ataxia, signs of progressive autonomic failure, significantly limiting human life.
- Prolonged temporary disability with an unfavorable or questionable prognosis regarding the restoration of sensory, motor and other functions.
- Periodic relapses and progressive course of the disease, taking into account its origin, the development of postherpetic neuralgia.
- Inability to return to work in the specialty, due to a motor defect and/or working conditions that are contraindicated for a particular person, if they cannot be changed by the conclusion of the commission.
Mechanism of development of polyneuropathies
Diagnostics
The primary symptoms of polyneuropathy are identified by a neurologist during the examination of the patient. The next stage of diagnosis is laboratory tests. A child or adult is prescribed general clinical and biochemical blood tests, genetic and immunological tests. In some cases, a biopsy specimen is taken from a peripheral nerve. Histological analysis of the obtained material will allow doctors to determine the nature of damage to the nerve fibers.
Electromyography remains the key hardware method for diagnosing polyneuropathy. During the procedure, doctors examine the bioelectric potentials of the patient's muscles. EMG allows you to assess the intensity of the pathological process and exclude other neurological diseases from the history of a child or adult.
According to the speed of development, they are distinguished:
- acute polyneuropathies develop in less than four weeks: the most common is Guillain-Barré syndrome;
- subacute polyneuropathy is called if the disease develops over 4-8 weeks;
- chronic course, when the disease develops for more than two months (8 weeks). The chronic course is more typical with alcoholism, uncompensated diabetes mellitus, vegetarianism, or simply poor nutrition leading to a deficiency of B vitamins, hereditary causes, inflammatory processes involving autoimmune mechanisms that primarily affect the myelin sheath of the nerves, etc.
In addition to the above, the cause of this disease can be: the use of neurotoxic drugs, chemotherapy, intoxication with salts of heavy metals, some chronic somatic diseases with symptoms of liver and kidney failure, diseases of the endocrine organs, for example, the thyroid gland.
Polyneuropathy also occurs in autoimmune diseases, both affecting predominantly peripheral nerves (Guillain-Barré syndrome, multifocal motor neuropathy, chronic inflammatory demyelinating polyneuropathy, paraproteinemic polyneuropathy) and systemic connective tissue diseases (rheumatoid arthritis, Sjögren's disease, systemic lupus erythematosus).
In the development of polyneuropathy, infectious factors of both a bacterial nature (borreliosis, botulism, diphtheria, syphilis, HIV infection) and viral (influenza, cytomegalovirus, herpes, etc.) often play an etiological role. A special place in the group of polyneuropathies is occupied by hereditary neurodegenerative diseases, such as transthyretin familial amyloid polyneuropathy, polyneuropathy with porphyria, hereditary neuropathy with a tendency to paralysis from compression.
Treatment
Therapy for hereditary forms of polyneuropathy is symptomatic. Autoimmune disorders are brought into remission. Diabetic, alcoholic and uremic types of pathology require a combination of drug therapy and exercise therapy. Against the background of diphtheria polyneuropathy, patients are prescribed artificial ventilation.
Modern pharmacology cannot offer people suffering from polyneuropathies drugs that effectively eliminate damage to the cylindrical processes of nerve cells or myelinated nerve fibers. Palliative therapy includes vitamin complexes and neurotrophic agents.
Treatment of porphyritic polyneuropathies involves the use of glucose and painkillers. In the demyelinating form of the disease, patients are prescribed membrane plasmapheresis, supplemented by immunoglobulin injections. If they are insufficiently effective, doctors use glucocorticosteroids - prednisolone or its analogues.
Treatment of diabetic polyneuropathy of the lower extremities is carried out not only by a neurologist - the patient needs to consult an endocrinologist. For severe pain, men and women receive tricyclic antidepressants, pregabalin and gabanetin.
The symptoms of toxic polyneuropathies become less obvious when contact of a child or adult with the toxin is excluded. In case of drug intoxication, the doctor adjusts the daily dose received by the patient.
Surgical intervention against the background of polyneuropathy is performed when there is deformation of the feet and the development of joint contractures. In this case, the patient will face a long period of rehabilitation. To treat uremic polyneuropathy, a child or adult may require a kidney transplant.
Neuropathy Treatment Basics
This disease must be treated comprehensively, always with correction of the underlying pathology. For autoimmune diseases, hormones and cytostatics are prescribed; for diabetes, glucose-lowering medications or insulin; for the toxic type of disease, cleansing techniques (hemosorption, plasmapheresis).
The goals of treatment for lower extremity neuropathy are:
- restoration of nervous tissue;
- resumption of conductivity;
- correction of disorders in the circulatory system;
Advertising: - improvement of well-being;
- reduction of pain and other disorders;
- optimization of motor function of the legs;
- increasing metabolic rate.
There are many treatment methods, the main one being medication.
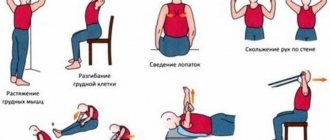
Surgical treatment is practiced only in the presence of tumors, hernias, and after injuries. To prevent muscle atrophy, all patients are recommended to exercise from a special exercise therapy complex; at first, they are performed under the supervision of a rehabilitation physician.
If you have neuropathy, you should follow a diet with an increase in the content of vitamins B, and you should also exclude alcohol, foods with chemical additives, marinades, fried, and smoked foods.
The disease is successfully treated with physiotherapy. Massage, magnetic therapy, therapeutic mud, reflexology, and electrical muscle stimulation have proven themselves to be excellent. To prevent the formation of ulcers, you should wear special shoes and use orthoses.
Basic drugs for the treatment of pathology
In the treatment of neuropathy, drugs play a leading role. Since the basis is degeneration of nerve tissue, the structure of the nerve roots should be replenished with medication. This is achieved by using the following medications:
Advertising:
- Neuroprotectors
, metabolism accelerators in nerve cells - Piracetam, Mildronate. They improve the trophism of nervous tissue, helping to improve its structure. - Anticholinesterase drugs
- Ipidacrine, Prozerin. They optimize the sensory functioning of nerves and help in the transmission of nerve impulses. - Antioxidants
— Mexidol, Cytoflavin. They help to “quench” the action of free radicals, which aggravate the destruction of peripheral nerves. - Alpha lipoic acid preparations
. They enhance metabolism, enhance the restoration of neurocytes, especially indicated for diabetic neuropathy.
B vitamins are mandatory in the course of therapy, especially B12, B6, B1. Most often, combined drugs are prescribed - Neuromultivit, Milgamma in tablets, injections. After taking them, sensitivity disorders are eliminated, all symptoms are reduced in severity.
What else is used to treat neuropathy?
Advertising:
Vitamins that are powerful antioxidants are very useful for the body in any form of neuropathy of the lower extremities - ascorbic acid, vitamins E, A. They are necessarily used in complex therapy of the disease to reduce the destructive effects of free radicals.
In case of severe muscle spasms, the patient will be helped by muscle relaxants - Sirdalud, Baclofen, which are used only with a doctor's prescription - if abused, they can increase muscle weakness.
There are other medications against this pathology. They are selected individually. These are:
- hormonal agents to suppress pain and inflammation - Prednisolone, Dexamethasone
; - vascular medications to improve blood circulation in tissues - Pentoxifylline, Trental
; - antidepressants, sedatives, drugs against excessive anxiety - Amitriptyline, Duloxetine
; - analgesics, including narcotics for severe pain - Tramadol, Analgin, as well as non-steroidal anti-inflammatory drugs - Ketoprofen, Ketonal Duo
.
Locally it is recommended to use ointments with novocaine, lidocaine, non-steroidal anti-inflammatory drugs, as well as warming ointments with red pepper and animal poisons. In case of bacterial infection of the skin of the feet and legs, bandages with antibiotics are applied (Tetracycline ointment, Oxacillin).
Traditional treatment of neuropathy
Treatment with folk remedies is used with caution, especially for diabetes. Recipes can be like this:
- Combine the yolk of a raw egg and 2 tablespoons of olive oil. Add 100 ml of carrot juice, a tablespoon of honey. Drink 50 ml three times a day after meals. Course - 14 days.
- Pour 2/3 cup of 9% vinegar into a bucket of warm water, add a glass of salt. Soak your feet in the water for 15 minutes. The course is once a day for a month.
- Mix 3 tablespoons of fenugreek seeds, a tablespoon of bay leaf, chop everything. Brew a liter of boiling water in a thermos for an hour, then drink in small portions throughout the day. Course - 10 days.
- Dilute blue clay with water to a paste and apply to feet. Let it dry, then rinse off. Repeat every day for 2 weeks.
With timely treatment, the disease has a good prognosis. Even if the cause of neuropathy is very severe, it can slow or stop its progression and improve a person's quality of life.
Forms of alcoholic polyneuropathy
They differ in the set of symptoms and clinical picture.
Sensory. It manifests itself as pain in the hands, feet (the latter is more common), numbness or burning, cramps, and a feeling of chilliness. The sensitivity of the palms to temperature or pain stimuli may become more acute or, conversely, reduced. Sensory disturbances may be segmental. They often occur together with noticeable vegetative-vascular disorders (sweating, marbling of the skin on the feet or palms, or a change in its shade to bluish). A number of reflexes may decrease.
Motor. Sensory disturbances in this form are mild, most often affecting the legs. The muscles are weakening. The tibial nerve is often affected, causing the flexion of the feet and toes to be impaired, a person may walk on their toes, and the extensors of the foot and toes do not work properly. In the area of the legs and feet, muscle atrophy may develop or increased muscle tone may appear. Without treatment, paresis (muscle weakness) intensifies to the point of paralysis, pain, numbness, and sensory disturbances may appear. The same symptoms can occur in the hands.
Ataxic. Coordination of movements and gait are impaired due to a decrease in deep sensitivity. A feeling of numbness appears in the legs, the feet and palms become numb, and reflexes are reduced.
Mixed. With this form of polyneuropathy, there are symptoms characteristic of the motor and sensory forms: pain, decreased sensitivity, weakness of the muscles of the arms and legs, changes (usually decreased) reflexes.
Alcoholic polyneuropathy can be asymptomatic (almost all patients with chronic alcoholism have it). In the chronic form, symptoms increase gradually over a year or more. In acute or subacute form, it develops over several weeks and is more pronounced.
Order alcoholism coding
Experienced doctors and narcologists. In hospital or at home. 24-hour service in Moscow and the region. Professional, anonymous, safe.
Diseases of the peripheral nervous system in the elderly, despite their high frequency, are one of the least studied issues in neurogeriatrics. Their prevalence in patients under 55 years of age ranges from 3.3 to 8%, and in older age groups (60–74 years) reaches 22% [1]. The vast majority of them are patients with diabetic polyneuropathy (DNP). In the elderly, specific forms of PSP are rare, but certain forms are more common and have their own clinical characteristics in comparison with younger patients. This is due to age-related changes occurring in the body, which lead to a decrease in the density of the gray matter of the brain and spinal cord, a decrease in the amount of neurotransmitters, hypothalamic-pituitary insufficiency, hormonal disorders, as well as disorders of other types of metabolism. Thus, in the elderly there is a decrease in sensory functions of all modalities (hearing, vision, smell, taste and sensitivity). Age-related changes also occur in the peripheral nervous system in the form of a decrease in the number of nerve fibers, primarily thick myelinated fibers, their partial demyelination, and a decrease in the distance between nodes of Ranvier [2, 3]. Degenerative processes in old age are observed not only in the myelin sheath of peripheral nerves, but also in axons. Wallerian degeneration occurs, a slowdown of the axoplasmic flow, which leads to disruption of muscle trophism. The number and size of sensory neurons in the dorsal root ganglia also decrease; the fibers that conduct vibration sensitivity are most affected. In this regard, a decrease in Achilles reflexes and the threshold of vibration sensitivity in the feet in people over 70 years of age is considered normal, while in young patients it is a sign of PSP. A decrease in the number of peripheral fibers with age, signs of demyelination and neurogenic inflammation resemble pathological changes in PSP. Electroneuromyographic studies (ENMG) show lower rates of excitation propagation compared to young people. Thus, in 70-year-old people, HRV is 15-25% less compared to 30-year-old people [2].
Restoration of nerve function in elderly patients with PSP occurs more slowly than in younger patients, which is associated with a decrease in metabolism and regeneration processes in peripheral nerves, deterioration of blood supply and trophic disorders. This is due to the presence of concomitant diseases such as arterial hypertension (AH), atherosclerosis, etc.
In old age, the following PNPs are most common: 1) dysmetabolic (diabetic, with vitamin B12 deficiency, diseases of the thyroid gland, liver, kidneys); 2) toxic (alcohol); 3) autoimmune (chronic inflammatory demyelinating, against the background of monoclonal gammopathy), 4) paraneoplastic; 5) amyloid; 6) idiopathic small fiber PSP.
When evaluating an elderly patient with PSP, a differential diagnosis should always be made with paraneoplastic PSP. Symptoms of PSP can occur either after the onset of clinical symptoms of the tumor or before them. They are most often found in cancer of the lung, gastrointestinal tract, kidney, uterus and appendages, thyroid gland and blood diseases. Differences in the development, course and prognosis of most PSP in old age compared to young patients are more frequent damage to the autonomic nervous system; the presence of neuropathic pain syndrome, which in the elderly requires quick and adequate relief; instability when walking and falling, which are of a polyetiological nature; difficulties in selecting effective therapy due to concomitant diseases.
Damage to the autonomic nervous system is most common in diabetic, alcoholic and amyloid PSP. Their severity in elderly patients is aggravated by neurodegenerative processes in the brain with damage to autonomic structures (see table). The most life-threatening is cardiac autonomic neuropathy (CAN). According to a 6-year follow-up of patients with diabetes mellitus (DM), the mortality rate among them in the presence of CAN reaches 29%, while the mortality rate among patients with DM without CAN is only 6% [4]. One of the most severe symptoms of CAN, worsening the quality of life of patients, is orthostatic hypotension. A feature of diabetic PSP in elderly patients with diabetes is the high frequency of hypertension, leading to an increase in the incidence of CAN. Thus, in patients with type 2 diabetes without hypertension, the prevalence of CAN is 48%, while the combination of diabetes and hypertension leads to the development of CAN in 79% of cases [5]. This leads to a labile course of hypertension, so in patients with CAN at night there is a sharp increase in blood pressure (BP) or, conversely, there is no decrease in blood pressure, which is characteristic of the night time. These factors increase the likelihood of developing stroke and myocardial infarction, so patients with hypertension and diabetes are considered to be at high risk of cardiovascular complications.
Damage to body systems in autonomic neuropathy
To prevent severe consequences of CAN, its early diagnosis is important in order to begin pathogenetic treatment as early as possible. For this purpose, special diagnostic tests have been developed - a deep breathing test (change in heart rate when breathing at a frequency of 6 times per minute); Valsalva, Shelong tests (orthostatic test), “30:15” test (heart rate increases when standing up with a maximum value by the 15th beat, followed by a decrease with a minimum value by the 30th beat); test with isometric load [6]. For early detection of CAN, cardiovascular tests are performed in patients with type 1 diabetes 5 years from the onset of the disease, and in patients with type 2 diabetes immediately upon diagnosis and then once a year.
One of the pressing problems of gerontology is the instability of patients when walking, leading to falls. According to WHO, falls are the second leading cause of injury and death from accidents. It has been shown that with age, the state of balance maintenance systems (the frontal lobe and its connections, the vestibular apparatus, the cerebellum and its connections, proprioception) deteriorates. In patients with PSP, unsteadiness when walking and the risk of falls depend on the extent of damage to thick myelinated nerve fibers, resulting in sensory ataxia. It is more pronounced in patients with demyelinating PSP. However, diabetic PSP with damage to thick fibers, being predominantly an axonal variant, is also an independent factor of imbalance [7].
Neuropathic pain is observed in diabetic, alcoholic, paraneoplastic, amyloid PSP. The pain can be very severe, which leads to a significant decrease in the quality of life of patients. The mechanisms of development of pain syndrome and aging are similar [8–10]. In both conditions, changes in neurotransmitter synthesis and receptor expression in the spinal cord are observed. A decrease in sensitivity in old age may be associated with neuroplastic changes at the level of the dorsal horn and functional disorders of the descending pathways of the antinociceptive system. At the cellular level, aging triggers mechanisms that affect the functioning of sensory neurons: a decrease in the expression of genes encoding neurotrophic factors, neuropeptides, intracellular messengers, mitochondrial proteins, as well as ion channels and membrane receptors. The synthesis of calcitonin gene-related peptide, substance P, and nitric oxide in the cells of the dorsal horns of the spinal cord decreases. With age, the density and functional activity of glial cells of the spinal cord, which perform trophic functions, decrease. Taken together, the changes described above lead to disruption of the portal pain control at the spinal level.
Speaking about the pathogenesis of diseases of the peripheral nervous system, the role of channelopathies, a group of diseases caused by dysfunction of sodium, calcium, potassium, and chloride channels in the cell membrane, is actively discussed. Voltage-gated sodium channels, in addition to participating in the generation of action potentials, are involved in the formation and functioning of neuronal connections, starting from the early embryonic period of development. The mechanisms of the pleiotropic effects of sodium channels include reciprocal interactions between neurons and glia through neurotransmitters, growth factors, and cytokines. Channelopathies lead to the development of painful PSP and neurodegeneration (for example, in multiple sclerosis) [11].
Channelopathies are divided into primary or genetic (caused by mutations encoding the synthesis of glycoprotein ion channels) and secondary, resulting from dysfunction of ion channels in dysmetabolic, toxic or autoimmune diseases. These diseases cause changes in the transcription of ion channels, decreased expression of ion channel genes, and the opening of other channels that are normally inactive. There are 9 alpha subunits of voltage-gated Na channels (Na1.1-1.9). Each subunit has specific functions and varying abundance. Thus, Na1.7 and Na1.8 subunits are key molecules involved in the formation of peripheral neuropathic pain, increased pain sensitivity associated with inflammation and tissue damage. In painful diabetic PSP, suppression of the function of voltage-gated Na 1.6, Na1.7 and Na 1.9 channels is observed, which contributes to the development of allodynia and hyperalgesia.
Idiopathic small fiber neuropathy, which often occurs in the elderly, is also based on a mutation in the gene encoding the Nav1.7 channel glycoprotein. This form of neuropathy has a late onset; pain in most patients begins in the distal extremities; generalized pain syndrome is less common. Often such patients experience autonomic disorders. Some of the pain-related symptoms (psychomotor agitation, increased blood pressure, vomiting, hypercoagulation, hyperglycemia) are life-threatening in elderly patients with severe comorbid diseases. This dictates the need for timely and adequate pain therapy.
Currently available treatments for neuropathic pain, such as antiepileptic drugs (AEDs), tricyclic antidepressants (TCAs), and serotonin-norepinephrine reuptake inhibitors (SNRIs), act only on selected manifestations of neuropathic pain, and their long-term use is associated with an increase in the number of side effects : peripheral edema and weight gain (TCAs and AEDs from the gabapentinoids group), hypertension (TCAs and SSRIs) and associated orthostatic hypotension due to autonomic neuropathy and the use of antihypertensive drugs. If an elderly patient, in addition to pain, also has depressive disorders, prescribing TCAs and SSRIs is advisable not only to avoid polypharmacy, but also due to the fact that both of these disorders have common pathogenetic mechanisms - a decrease in the activity of the antinociceptive system due to age-related involution of descending serotonergic and noradrenergic pathways . If a patient with pain has anxiety disorders, it is optimal to use gabapentinoids (pregabalin, gabapentin) and gamma-aminobutyric acid derivatives - GABA (baclosan, noofen), which also affect the mechanisms of the vicious circle of pain-spasm-pain. Selective potassium channel activators (flupirtine) are also capable of influencing the mechanisms of pain and spasm, suppressing the excitability of neurons in the anterior and posterior horns of the spinal cord, but the hepatotoxicity of flupirtine limits its use in the elderly. Sodium channel blockers (carbamazepine and oxcarbazepine) have a lesser effect on anxiety and spasm mechanisms. Their analgesic effect is due to the suppression of hyperexcitability and ectopic activity of peripheral and central nociceptors by stabilizing neuronal membranes and reducing the readiness to produce an action potential. This mechanism causes class-specific side effects of AEDs - sedation, ataxia, decreased cognitive function, which is undesirable for elderly patients.
One of the safest drugs for the treatment of neuropathic pain in the elderly is gabapentin (gabagamma), which is structurally close to the inhibitory neurotransmitter GABA, a neurotransmitter involved in the transmission and modulation of pain. It is assumed that the central analgesic effect of gabapentin develops due to interaction with specific α2δ2 calcium channels, which leads to a decrease in the action potential of axonal membranes. Gabapentin increases the concentration of GABA in the cytoplasm of neurons and increases the content of serotonin in the blood plasma. It is able to penetrate membrane barriers using certain amino acid transport mechanisms, increase the concentration and, possibly, synthesis of GABA in the central nervous system, blocking pain at the spinal level, and prevent the death of neurons by inhibiting glutamate synthesis.
Gabagamma not only significantly reduces the severity of pain during painful PSP, but also improves the quality of life of patients, since the frequency of side effects (nausea, dizziness, drowsiness, tremor, nystagmus, dry mouth) is minimal due to the flexible dosage system of the drug, in contrast from other forms of gabapentin. Most forms of gabapentin available on the domestic pharmaceutical market have two dosages - 300 and 600 mg, providing gradual titration of the drug to an analgesic dose, however, an intermediate dosage of 400 mg, which allows optimizing the individual dose selection for analgesic therapy, is possible only when treating with gabapentin.
The analgesic effect of gabapentin in relation to both spontaneous and stimulus-dependent pain significantly exceeded the placebo effect in various forms of neuropathic pain syndromes, primarily in diabetic PSP; however, in the case when an elderly patient is prescribed only analgesic therapy without drugs that restore nerve function, pain disappears as a danger signal does not encourage the patient to continue treatment, therefore, isolated symptomatic treatment of pain ultimately leads to worsening sensorimotor deficits in the limbs, an increase in the manifestations of autonomic neuropathy, and the formation of autonomic-trophic disorders, which, if infected, can cause amputation of the limb. Thus, for elderly patients, pathogenetic therapy is necessary to help restore the function of peripheral nerves, and in some cases, improve the functions of the central nervous system. Such drugs are characterized by relief of the main manifestations of neuropathy - both its symptoms and functional indicators of the affected nerves [12, 13].
The role of oxidative stress in the aging of the nervous system and in the development of PSP is recognized as leading [14–18]. Oxidative stress is the process of cell damage caused by free radicals (FR). Most of them are constantly formed in the cell - about 5% of the oxygen consumed by tissues is converted into SR, but their level is normally so low that the cell either inactivates them using antioxidant systems (reduced glutathione, vitamins C and E, coenzyme Q, which neutralize short-lived SR, turning into long-lived or stable radicals in which the unpaired electron is delocalized - oxidized glutathione, ascorbate radical, tocopheroxy radical, coenzyme Q radicals) or replaces damaged molecules. Oxygen CP produced as by-products of normal metabolism in the mitochondrial respiratory chain, as well as other cytoplasmic reactions, does not cause cell damage, but CP levels exceeding its protective capabilities cause serious cellular damage (for example, ATP depletion) and, as a result, cell destruction. One of the least reactive SRs, superoxide, is converted into more aggressive ones (hydroxyl radical, etc.), which can cause oxidation and destruction of many cellular components - membrane proteins and lipids, DNA. Depending on the intensity of oxidative stress, cells can die as a result of apoptosis or necrosis. In necrosis, the cell membrane is destroyed and the contents of the cell are released into the intercellular space, which can result in damage to surrounding cells and tissues. The effect of oxidative stress depends on the severity of its severity. Cells can return to their original state with minor disturbances, but more pronounced oxidative stress causes cell death. Nevertheless, it cannot be considered as absolutely harmful to the body. In some cases, oxidative stress is used by the body as a defense mechanism. The immune system uses it to fight antigens, and some SRs serve as intermediaries (primary messengers) in signal transmission.
Various organs and tissues are to varying degrees susceptible to the action of CP and demonstrate different resistance during the implementation of oxidative stress - the neurons of the brain and spinal cord are the least resistant to it, and the maximum resistance is observed in skin cells (this is understandable, because keratinocytes live in borderline conditions , where the access of oxygen from the air is practically unlimited). That is why oxidative stress is the cause or an important component of such pathological conditions of the central and peripheral nervous system as diabetic and alcoholic PSP, radiculopathy and compression-ischemic mononeuropathies and plexopathies, cerebral ischemia, neurodegenerative diseases, and is also one of the stages of the aging process. Under conditions of oxidative stress, the synthesis of nitric oxide (NO), the main regulator of vascular wall relaxation, is inhibited, and nuclear factor (NF-kB) is activated, initiating the release of substances that impair blood flow, such as endothelin-1. In this regard, it seems reasonable to use drugs with antioxidant effects, thioctic or alpha-lipoic acid (thiogamma), for the pathogenetic treatment of neuropathies in elderly patients, the effectiveness and safety of which have been proven in a number of double-blind, randomized, placebo-controlled studies [13-18 ].
To activate regenerative processes in peripheral nerves, neurotropic B vitamins (B1, B6, B12) are used. Their simultaneous use stimulates axoplasmic transport of structural elements of the membrane or myelin sheath, such as choline. Thiamine promotes remyelination by activating phospholipase-A, which enhances the hydrolysis of fatty acid esters; in addition, by enhancing energy supply in the form of ATP, it supports axoplasmic transport, which is especially important for restoring the trophic function of nerves. Pyridoxine is involved in the synthesis of transport proteins and sphingosine, a structural element of the nerve fiber membrane and neurotransmitters of the antinociceptive system (serotonin, norepinephrine). Cyanocobalamin ensures the delivery of fatty acids to cell membranes and the myelin sheath. Its use promotes not only remyelination (due to the activation of the transmethylation reaction, which ensures the synthesis of phosphatidylcholine in nerve cell membranes), but also a decrease in the intensity of pain, which is associated with the intrinsic antinociceptive effect of high doses of cyanocobalamin. Neurotropic complexes of B vitamins (milgamma) increase the speed of nerve impulses and improve reparative processes in the peripheral nerve. It is advisable to begin therapy for elderly patients with milgamma injections (2 ml intramuscularly for 10 days) due to the frequent presence of malabsorption syndrome in patients with PSP, caused by damage to the autonomic fibers that regulate the activity of the gastrointestinal tract. At the same time, patients can receive 600 mg of thiogamma-turbo intravenously for 15 days. Parenteral administration ensures maximum bioavailability of antioxidants and neurotropic vitamins in patients with malabsorption. After completing the course of injections, it is necessary to continue therapy with tablet forms (milgamma-compositum 1 tablet 3 times a day after meals and thiogamma 600 mg 1 tablet in the morning 30 minutes before meals) for 2-6 months, depending on the severity of PSP and the speed of recovery clinical and neurophysiological indicators.
Antioxidants and B vitamins have the highest evidence base in the treatment of neurological complications of diabetes and alcoholism, but nevertheless their potential in the pathogenetic treatment of other forms of damage to the peripheral nervous system in elderly patients requires further study. Sometimes the beginning of pathogenetic therapy leads to an increase in pain due to increased peripheral afferentation along regenerating nerve fibers, and the pain syndrome with monotherapy with B vitamins, anticholinesterase drugs or antioxidants regresses more slowly than with symptomatic therapy with TCAs, SSRIs or AEDs, therefore, with severe neuropathic pain syndrome At the beginning of treatment, it is advisable to prescribe combination therapy. The presence of orthostatic hypotension may limit the use of TCAs and require the addition of symptomatic therapy. For the treatment of orthostatic hypotension, mineralocorticoids are used (fludrocortisone acetate 0.1-1.0 mg per day). If fludrocortisone is ineffective or cannot be used as monotherapy, sympathomimetics (ephedrine, phenylpropanolamine, phenylephrine, methylphenidate, dextroamphetamine, tyramine, midodrine, yohimbine, DL-dehydroxyphenylserine) are prescribed. Synthetic analogues of vasopressin (desmopressin acetate, lysine vasopressin), erythropoietin, caffeine and cyclooxygenase inhibitors (indomethacin, flurbiprofen, ibuprofen) are considered additional drugs not used as monotherapy. There are non-pharmacological treatments for orthostatic hypotension, such as slow, gradual changes in body position; avoiding tension, straining and isometric exercises; the use of compensatory physical exercises; discontinuation of antihypertensive drugs; sleep with the head raised; eating a high-sodium diet and eating small portions; special overalls that compress the lower body; increased fluid intake (except for patients with heart and kidney failure) [3, 5].
The main requirement for the pathogenetic treatment of neuropathies in elderly patients is a reduction in pain symptoms while improving the functional parameters of peripheral nerves. Monitoring the effectiveness of therapy should include assessment of symptoms and clinical manifestations of neuropathy, as well as measurement of objective parameters of nerve function in ENMG studies and tests for cardiac autonomic neuropathy. When treating PSP, it is recommended to conduct neurophysiological studies 3 months after the start of pathogenetic therapy. When objective indicators of peripheral nerve function and autonomic innervation are normalized, treatment can be suspended. Monitoring of neurological deficit in such patients should be carried out at least once a year to decide on the need for repeated pathogenetic therapy.