Neurologist
SEMENOVA
Olga
5 years experience
neurologist, head of the office for diagnostics and treatment of cognitive disorders
Make an appointment
Polyneuropathies (polyneuropathies) are a group of pathologies affecting peripheral nerves. The clinical picture is determined by the form of the disease diagnosed in the patient. Specific signs are muscle weakness and atrophy, decreased tendon reflexes, sensory disturbances, and autonomic disorders. Treatment of polyneuropathy is carried out by a neurologist. The main task of the doctor is to determine the cause of the disease. Therapy is symptomatic.
Causes
The reasons leading to the development of polyneuropathy in patients are varied. Often, damage to nerve cells develops against the background of severe poisoning with methyl alcohol, arsenic or carbon monoxide. Similarly, axonal health can be affected by volatile compounds high in phosphorus.
In some cases, the symptoms of the pathology appear against the background of chronic intoxication of the human body, diphtheria, vitamin deficiency or diabetes mellitus. The disease can develop with uremia, liver cirrhosis, kidney damage, hypothyroidism or cancer. Long-term use by the patient of amiodarone, isoniazid or metronidazole can lead to the manifestation of polyneuropathy.
Classification of the disease
There are several classifications of polyneuropathies. If we consider the mechanism of nerve damage itself, the disease can take the following forms:
- axonal polyneuropathy – axonal dysfunction occurs. This form is considered the most severe;
- demyelinating – the structure of the myelin sheath of nerve fibers is disrupted;
- neuropathic - lesions directly affect the bodies of nerve cells.
Based on etiology, polyneuropathies are divided into:
- toxic;
- hereditary;
- post-infectious;
- paraneoplastic;
- dismetabolic;
- polyneuropathies arising against the background of systemic diseases of the body;
- idiopathic.
Depending on which nerves are damaged, polyneuropathies can be sensory, motor, autonomic, or mixed.
Kinds
Neurologists distinguish two types of polyneuropathies based on pathogenetic signs of damage to peripheral nerves - axonal and demyelinating. The first form of the disease affects the long, cylindrical extensions of nerve cells called axons. The second provokes the loss of myelin in myelinated nerve fibers. Late stages of axonal disorders are accompanied by demyelination. Primary demyelinating polyneuropathies are complicated by secondary damage to the cylindrical processes of nerve cells.
In the process of diagnosing and treating polyneuropathies, doctors may use other classifications of the disease. Based on the clinical picture, neurologists distinguish motor, sensory and autonomic polyneuropathies. In their pure form, these types of pathology are rare: most patients are diagnosed with damage to two or three types of nerve fibers (motor-sensory, sensory-vegetative, etc.).
Studying the etiology of the disease allows neurologists to identify hereditary, autoimmune, metabolic, nutritional, toxic and infectious-toxic polyneuropathies.
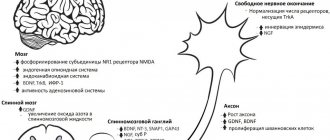
Polyneuropathy (PNP) is a disease of the peripheral nervous system that develops as a result of diffuse damage to peripheral nerves—their axons (axonal polyneuropathies), the myelin sheath (demyelinating polyneuropathies) or the bodies of neurons (neuronopathies) [9]. The pathogenesis of axonal type polyneuropathies is based on generalized damage to the axial cylinders of peripheral nerves.
Pathogenesis
.
Axonal degeneration (axonopathy) is the result of impaired neuron metabolism due to insufficient energy production in mitochondria and/or impaired axonal transport. In axonopathies, the myelin sheath suffers secondarily (secondary demylinization). Myelin can be damaged as a result of nerve ischemia (damage to the vasa nevrorum
), deposition of substances or immune complexes toxic to the nerve in the endoneurium (which is typical, for example, of diabetes mellitus, especially type 1 - with high hyperglycemia and autoimmune disorders) [4]. Accordingly, among the causes of axonopathy of peripheral nerves are metabolic disorders, toxic effects, ischemia of nerve trunks, hereditary predisposition and autoimmune mechanisms [5]. According to the etiological factor, the vast majority of axonal PNPs are metabolic and toxic, among which the first place is occupied by diabetic (up to 50% among patients with diabetes mellitus and up to 90% of damage to the nervous system in diabetes [9, 12]) and alcoholic (up to 50% among patients alcoholism [2, 9]).
The cause of toxic-metabolic PNP is exo- and endogenous intoxication. Substances coming from outside or their own metabolites that are toxic to peripheral nerves cause their damage. The severity of this damage depends on the degree of toxicity of the agent (acute metabolic PSP is distinguished, developing against the background of severe general intoxication, for example, PSP with rapidly increasing liver failure or uremia, poisoning with organophosphorus substances, arsenic, lead, as an undesirable effect of treatment with lithium drugs, cytostatics ), the duration of its influence, the own genetic characteristics of the metabolism of nervous tissue, and the autoimmune factor also plays an important role [13].
Most of the metabolic ASPs frequently encountered in the clinic are the result of long-term exposure to endo- and exogenous toxic agents (diabetic, hepatic, uremic, alcoholic, occupational, medicinal). The result of such intoxication is damage to the axial cylinder of the axon. Clinically, this is manifested not only by sensory disturbances and muscle weakness (which is also typical for demyelinating PSP), but also by muscle wasting and pronounced trophic disorders. All these signs indicate long-term suffering of peripheral nerve axons. Electroneuromyography (ENMG) reveals a decrease (sometimes to the point of complete absence) in the amplitude of sensory potentials and M-responses of peripheral nerves. In patients with axonal type PSP, motor functions are often sufficiently preserved (there are no pronounced paresis, patients retain the ability to walk, often without additional support), while sensory (pain and paresthesia) and trophic disorders are disabling. Thus, axonal sensorimotor PSP is one of the important pathogenetic factors in the development of diabetic foot syndrome [3]. Axonal nerve damage develops slowly and gradually, but with proper treatment it is potentially reversible.
With massive exposure to an agent toxic to the peripheral nervous system, the participation of the ischemic component (due to the suffering of the vasa neurorum
), autoimmune influences develop PNP of the axonal demyelinating type, such as uremic, lead, amiodarone, caused by exposure to substances highly toxic to nerves [9]. In the most common diabetic PSP, the demyelinating component is maximally present in insulin-requiring diabetes mellitus (characterized by higher hyperglycemia), and the severity of demyelination of peripheral nerves increases with sharp increases in blood glucose levels [4, 7]. The presence of the demyelinating component in metabolic PSP is influenced by hereditary factors (genetically determined myelinopathy, which would proceed subclinically without additional exposure to a toxic agent), autoimmune damage (for example, myelinopathy is more pronounced in type 1 diabetes mellitus, in which immunocompetent cells damage pancreatic tissue ) [4, 14].
PSP of the axonal demyelinating type are more severe, with severe paresis, sensory ataxia, and neuropathic pain syndrome, but often, with timely elimination of the toxic factor, they quickly regress. In case of a very severe and persistent course of the disease, given the role of the autoimmune mechanism in the development of this type of metabolic PSP, short-term administration of immunomodulatory therapy (usually glucocorticoids, sometimes cytostatics) is necessary [9, 11].
Clinical course of PSP.
As already mentioned, with prolonged exposure to an agent that is moderately toxic to nerves, axonopathy develops slowly, which is not always noticeable. Predominant damage to poorly myelinated vegetative and sensory fibers causes paresthesia, increased cold sensitivity of the hands and feet, and mild trophic disorders. With ENMG, it is not always possible to identify characteristic signs of sensory and motor axonopathy - a decrease in the amplitudes of sensory potentials and M-responses - if large, richly myelinated nerves are examined, the axons of which suffer later. Signs of secondary myelinopathy are revealed more quickly, both clinical - the addition of neuropathic pain, paresis, and myographic - a decrease in the speed of propagation of excitation (SRV) along the nerves.
Provided an adequate therapeutic strategy is eliminated the effect of the factor damaging peripheral nerves, the prescription of drugs that affect the metabolism of nervous tissue, remyelination occurs, which is reflected both in ENMG in the form of an increase in SRV, and clinically in the form of regression of paresis and neuropathic pain, improvement in sensitivity. However, if polyneuropathy developed under conditions of prolonged action of a toxic agent, damage to the axons of peripheral nerves, a decrease in the amplitudes of sensory potentials and M-responses to ENMG persists, since the axial cylinders of the nerves have a much lower ability to regenerate than myelin.
Thus, in a study [8] devoted to the treatment of alcoholic PSP, the leading ENMG sign of damage to peripheral nerves in most patients was a decrease in ERP. Under conditions of elimination of the toxic factor (in this case, alcohol intake) and treatment with α-lipoic acid (berlithion in a daily dose of 600 mg), patients quickly, within 1 month, regressed ENMG and clinical signs of myelinopathy - the severity of paresis and pain syndrome (in points on a visual analogue scale from 5.2±1.0 to 2.6±0.5) significantly ( p
<0.05) SRV increased (for motor fibers
n. tibialis
from 36.93±1.12 to 42.22±0.8 m/s, for sensory fibers
n. suralis
from 32.45±0.70 to 38 .24±0.50 m/s), but signs of axonopathy (decrease in the amplitudes of sensory and motor responses of peripheral nerves, sensory loss of the polyneuritic type, trophic disorders) persisted even after 6 weeks of treatment.
Thus, we can imagine the following scheme for the development and course of metabolic PSP (see table)
: highly toxic, highly concentrated toxic agents lead to the development of polyneuropathy of the axonal demyelinating type (in the development of which hereditary, vascular, and autoimmune factors also play a significant role), long-acting endo- and exogenous poisons lead predominantly to axonal suffering with moderately severe secondary myelin damage (so how the process of remyelination constantly occurs) [7].
Treatment.
Axonal damage to nerves, both primary and secondary, is reversible due to the regeneration of damaged axons and the terminal sprouting of surviving axons, but this process proceeds slowly (months), often axonal regeneration is incomplete. To regress axonopathy of peripheral nerves, it is necessary to neutralize the effects of the toxic agent (correction of metabolic and nutritional disorders, detoxification, influence on autoimmune mechanisms); pathogenetic therapy aimed at restoring impaired axonal metabolism (mitochondrial disorders, damage caused by oxidative stress) is also of great importance.
Thus, in the pathogenetic treatment of axonal PSP, depending on their nosological and clinical-electrophysiological variant, detoxification and correction of metabolic and nutritional disorders should be present; vascular therapy (disaggregants, venotonics, pentoxifylline, vasoprostan); drugs that affect the universal mechanisms of axonal damage (vitamins and vitamin-like drugs); if necessary, influence on autoimmune components (glucocorticoids, plasmapheresis). In some cases, when the representation of myelinopathy in the structure of damage to peripheral nerves is large and it is difficult to distinguish toxic-metabolic polyneuropathy from inflammatory (for example, Guillain-Barré syndrome in a patient with alcoholism or the debut of chronic inflammatory demyelinating PSP in a patient with diabetes), it is necessary to conduct a trial of immunomodulatory therapy , its rapid effectiveness will allow us to speak in favor of the prevalence of the autoimmune mechanism of damage to peripheral nerves in the patient [7, 11].
In the treatment of all metabolic PNP, it is necessary to eliminate (if possible) the poison that affects the peripheral nerves and use drugs that improve the metabolism of the nervous tissue of the peripheral nerves. The latter must be used for a long time, since the first effect of these drugs will be to accelerate remyelination, but the restoration of the axons themselves - and these APNs are axonal - requires a significantly longer amount of time.
α-lipoic (thioctic) acid is a vitamin-like substance that is endogenously formed in the body; as a coenzyme, it participates in the oxidative decarboxylation of α-keto acids. The main function of endogenous lipoic acid in the body is to participate in the aerobic metabolism of the glycolysis product pyruvate. Thioctic acid is a coenzyme in the oxidative decarboxylation of pyruvic acid to acetyl-CoA and α-ketoglutaric acid to succinyl-CoA in the Krebs cycle. By facilitating the conversion of lactic acid into pyruvic acid with subsequent decarboxylation of the latter, α-lipoic acid helps eliminate metabolic acidosis [6]. Thioctic acid has a complex complex effect: hypoglycemic, lipotropic, hepatoprotective, anti-atherosclerotic, and is a powerful antioxidant. Lipoic acid can exist in oxidized (-SS-) and reduced (SH-) forms, due to which its coenzyme and antioxidant functions are realized. The reduced form, dihydrolipoic acid, serves as an electron donor for the restoration of other antioxidants (vitamins C, E and glutathione), and recycles vitamin E when it is depleted. Dihydrolipoate increases intra- and extracellular levels of glutathione, an endogenous antioxidant.
The effect of exogenously administered α-lipoic acid on polyneuritic syndrome was first discovered in diabetes mellitus. Hyperglycemia caused by diabetes mellitus leads to the deposition of glucose on the matrix proteins of blood vessels and the formation of advanced glycosylation end products, resulting in a decrease in endoneural blood flow and endoneural ischemia. α-lipoic acid leads to a decrease in blood glucose levels and an increase in glycogen content in the liver, and has a hypoglycemic effect. The effect of the drug reduces the severity of sensory symptoms of polyneuropathy - pain, burning, numbness and “crawling” in the limbs.
The use of α-lipoic acid has a positive effect on universal mechanisms of axonal damage, such as the damaging effects of oxidative stress and mitochondrial dysfunction, due to its antioxidant effect and increasing glutathione content. The energy-correcting effect of α-lipoic acid, which is tropic specifically to nerve axons, ultimately promotes rapid axonal regeneration [18].
The drug not only reduces the manifestations of oxidative stress, but also affects the vascular component of peripheral nerve damage, normalizing endoneurial blood flow (which is of great importance, for example, in diabetic microangiopathy) [16, 17]. The complex mechanism of action of α-lipoic acid explains its effectiveness against all axonal PNP, the pathogenesis of which is associated with toxic-dysmetabolic and vascular factors. Thus, the effectiveness of thioctic acid has been shown in uremic and alcoholic PSP, and in damage to peripheral nerves induced by cytostatics [15]. For the regeneration of peripheral nerve axons against the background of toxic and metabolic influences, the detoxification and hepatoprotective effects of α-lipoic acid are also important. The positive effect of the drug was noted in relation to liver diseases, hepatic coma, and some intoxications, including alcohol [6, 10].
With significantly severe axonopathy, given the slow rate of axon regeneration, long-term use of fairly high doses of α-lipoic acid is necessary. Usually its daily dose is 600 mg. It is preferable to start treatment with an intravenous drip of the drug - 600 mg (24 ml of solution) diluted in 200 ml of physiological solution, the duration of the infusion is from 2 to 4 weeks, depending on the severity of PSP. In especially severe cases, the drug is administered intravenously at a dose of 1200 mg per day. Then they switch to oral administration of α-lipoic acid - in tablets of 600 mg for at least 2 months [1].
In accordance with this clinical need - the need for long-term administration of fairly high doses of α-lipoic acid, given the potentially reversible, but slow nature of axonal regeneration - pharmaceutical forms of the drug have been developed. For example, Berlition 300 and Berlition 600, produced in the form of a solution for intravenous infusion of 12 and 24 ml, respectively (for the initial stage of treatment of PSP), and tablets of 300 and 600 mg (for continued therapy).
Compliance with all conditions of pathogenetic treatment of PSP - normalization of glycemia, cessation of the intake of exogenous poison, detoxification, correction of autoimmune disorders, long-term use of drugs that normalize axonal metabolism, promotes gradual regeneration of axons, reducing the severity of sensory and motor disorders, pain, paresthesia, paresis. Further studies are needed to clarify the dynamics of various types of metabolic ANPs against the background of such a therapeutic approach.
Symptoms of pathology
During the examination of the patient, the neurologist identifies signs of damage to motor, sensory and autonomic fibers. The degree of involvement of these structures in the pathological process determines the clinical picture of the disease. Thus, when occupational polyneuropathy is detected in men and women, neurologists record sensory-vegetative symptoms (which are associated with regular fixation of muscles in a state of static tension).
The predominance of motor signs of pathology becomes the cause of the development of paresis of the upper and lower extremities. In the absence of treatment, the patient's individual muscle groups atrophy, including the respiratory ones (against the background of Guillain-Barré syndrome). Later, tendon reflexes decrease, and the person has difficulty performing basic tasks such as fastening buttons or tying shoelaces.
Sensory symptoms develop symmetrically, affecting the feet and hands of patients in the early stages. Sensitivity disorder manifests itself in different ways. Patients may experience tingling, burning, numbness, and pain. In the later stages of the disease, adults and children experience disturbances in the pain and temperature sensitivity of the skin.
Autonomic disorders are accompanied by dry skin and dysregulation of vascular tone. Over time, patients develop signs of tachycardia and unstable functioning of the gastrointestinal tract. Men may experience erectile dysfunction.
Prevention of polyneuropathy of the lower extremities
In order to prevent a disease such as polyneuropathy of the lower parts of the body, it is necessary to stop drinking alcohol, regularly monitor blood and urine sugar levels, and when working with hazardous and toxic substances, it is imperative to use special personal protective equipment.
To avoid pain after relieving the disease, it is recommended:
- wear loose shoes that do not pinch your feet;
- do not take long walks over long distances;
- do not stand still for a long time without changing position;
- wash your feet in cool water.
Do not forget about physical therapy, designed to keep muscles in constant tone, preventing atrophy. Regular physical therapy for polyneuropathy will strengthen the body. It is worth leading a calm lifestyle, avoiding emotional stress, which can negatively affect the nervous system, eating right and listening to the state of the body in order to prevent the disease from returning.
Diagnostics
The primary symptoms of polyneuropathy are identified by a neurologist during the examination of the patient. The next stage of diagnosis is laboratory tests. A child or adult is prescribed general clinical and biochemical blood tests, genetic and immunological tests. In some cases, a biopsy specimen is taken from a peripheral nerve. Histological analysis of the obtained material will allow doctors to determine the nature of damage to the nerve fibers.
Electromyography remains the key hardware method for diagnosing polyneuropathy. During the procedure, doctors examine the bioelectric potentials of the patient's muscles. EMG allows you to assess the intensity of the pathological process and exclude other neurological diseases from the history of a child or adult.
Symptoms
Symptoms of polyneuropathy may include:
- Gradual onset of numbness and tingling in the legs or arms that may spread up the arms and legs
- Sharp stabbing or burning pain
- Increased sensitivity to touch
- Lack of coordination and falling
- Muscle weakness or paralysis if motor nerves are damaged
If autonomic nerves are involved, symptoms may include:
- Heat intolerance, as well as changes in sweating
- Digestive, bladder, or bowel problems
- Changes in blood pressure, which may cause dizziness
Treatment
Therapy for hereditary forms of polyneuropathy is symptomatic. Autoimmune disorders are brought into remission. Diabetic, alcoholic and uremic types of pathology require a combination of drug therapy and exercise therapy. Against the background of diphtheria polyneuropathy, patients are prescribed artificial ventilation.
Modern pharmacology cannot offer people suffering from polyneuropathies drugs that effectively eliminate damage to the cylindrical processes of nerve cells or myelinated nerve fibers. Palliative therapy includes vitamin complexes and neurotrophic agents.
Treatment of porphyritic polyneuropathies involves the use of glucose and painkillers. In the demyelinating form of the disease, patients are prescribed membrane plasmapheresis, supplemented by immunoglobulin injections. If they are insufficiently effective, doctors use glucocorticosteroids - prednisolone or its analogues.
Treatment of diabetic polyneuropathy of the lower extremities is carried out not only by a neurologist - the patient needs to consult an endocrinologist. For severe pain, men and women receive tricyclic antidepressants, pregabalin and gabanetin.
The symptoms of toxic polyneuropathies become less obvious when contact of a child or adult with the toxin is excluded. In case of drug intoxication, the doctor adjusts the daily dose received by the patient.
Surgical intervention against the background of polyneuropathy is performed when there is deformation of the feet and the development of joint contractures. In this case, the patient will face a long period of rehabilitation. To treat uremic polyneuropathy, a child or adult may require a kidney transplant.
Treatment at the Energy of Health clinic
Neurologists at the Energy of Health clinic will provide assistance with any form of polyneuropathy. Specialists will conduct a full diagnosis to accurately identify the extent and location of the lesion, and then prescribe treatment and rehabilitation in accordance with the individual characteristics of the body. We use comprehensive methods that include:
- drug therapy in accordance with indications and modern recommendations for the treatment of polyneuropathy, including courses of infusions;
- various types of physiotherapy: magnetic therapy, laser and ultrasound exposure, phonophoresis and electrophoresis;
- physical therapy under the supervision of an experienced instructor, training in exercises to restore limb function at home;
- massotherapy;
- sessions with a psychotherapist if you have depression, insomnia, or increased anxiety;
- organization of sanatorium-resort treatment for complete rehabilitation.