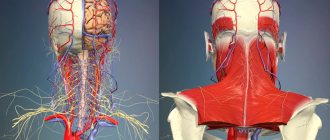
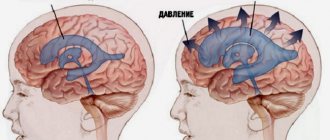
MRI of neck vessels Magnetic resonance imaging is one of the most modern and informative methods for diagnosing diseases of internal organs and soft tissues, including in the neck area. The procedure is actively used to assess the condition of the walls, the diameter of the lumens of blood vessels and their patency. One of the most important areas is the diagnosis of pathologies of the veins and arteries of the neck. The vessels of this area provide the functionality of the brain, upper spine, arms, and are also directly connected to the heart. Human life depends on their normal state. MRI of neck vessels can detect diseases in the early stages of its development. Using this technique, the doctor makes the correct diagnosis and prescribes treatment.
Neck vascular diseases
The largest arteries (left and right carotid), as well as their branches (internal and external), are located in this area. They provide 70% of the blood supply to the brain. The remaining 30% comes from the vertebral arteries, which rush deep into the skull through bony canals. The latter are formed by the transverse processes of the cervical vertebrae.
The large jugular veins take blood from the brain and face and carry it to the heart. The preservation of the functions of all of these vessels ensures continuous trophism of the head structures. Any damage to the veins and arteries is fraught with paralysis, loss of the ability to see or hear, impaired cognitive function and even death.
MRI of neck vessels allows you to diagnose:
- anomalies in the structure and location of veins or arteries;
- stenosis (narrowing of individual areas);
- pathological expansion (aneurysm);
- atherosclerosis (formation of cholesterol plaques on the walls) of arteries;
- inflammatory lesions (vasculitis);
- narrowing due to external pressure - a growing tumor, etc.;
- thrombosis and thromboembolism (blockage of the lumen with a blood clot);
- proliferation of scar tissue;
- dissection of the walls (dissection).
MR angiography (MRA) is used to assess the long-term consequences of injuries and to determine the causes of chronic and acute cerebral circulatory disorders. Often, the diagnosis of extracranial vessels is combined with a brain scan, which gives a more detailed picture of pathological changes.
1.3. Change in morphology
1.3.1. Hypoplasia
ICA hypoplasia: 0.079% Fig. 23 :
In contrast to agenesis, the thin vessel is identifiable. Again, skull base tomography is useful in visualizing the bony carotid canal, which is thinner than normal in hypoplasia [2].
Hypoplasia A1: 10% Fig.24 :
Asymmetry of A1 segments is observed in 80% of cases. Hypoplasia is defined when the vessel diameter is less than 1.5 mm [2].
Hypoplasia A2 (bihemispheric ACA): 7% Fig. 24 :
One of the A2 segments is hypoplastic. In this variant, the blood supply to the ipsilateral hemisphere occurs mainly from the contralateral (dominant) A2 segment [2].
Hypoplasia Pcom: 34% Fig.25 :
but complete absence is a rare finding [2].
Hypoplasia of the vertebral artery:
50% on the right side (left dominant), 25% on the left side (right dominant), 25% codominant. In approximately 0.2%, the vertebral artery ends in the PICA Fig. 12 and Fig. 26 [7] [2]
1.3.2. Hyperplasia
Hyperplasia of the anterior choroidal artery: 2.3% Fig. 27 :
The anterior choriodal artery arises from the posterior surface of the terminal segment of the ICA, distal to the origin of the Pcom. This is usually a small branch. If it is enlarged (hyperplastic), then it supplies blood to part of the territory of the posterior cerebral artery (occipitotemporal branch) [1, 2].
1.3.3. Early bifurcation (early division):
Early MCA bifurcation: This is a common finding Fig.28 :
The horizontal segment of the MCA is usually 12 mm long, but may be shorter, with early branching (bi- or trifurcation) [1].
1.3.4. Fenestration: 0.7% including all intracranial vessels Fig. 6 , Fig. 19 and Fig. 29 . Fenestration is the division of the lumen of an artery into two separate channels. Each canal has its own endothelium and muscle layer and can separate the adventitia. These two canals merge distally. Fenestration is most often observed in the posterior circulation [1, 2].
- Fenestration A1: 0-4% [1]
- Fenestration A2: 2% Fig. 19 [1]
- Acom fenestration: 12-21% [1]
- Fenestration of the vertebral artery Fig. 29 : 0.3-2% [1].
Fenestration of the basilar artery Fig. 29 : 0.12-1.33%: the basilar artery is formed by the fusion of two longitudinal neural arteries. Incomplete fusion results in segmental fenestration, which is usually present in the proximal segment of the basilar artery [2].
Fig. 23 CTA (a), hypoplastic ICA (arrows). CT bone window (b) asymmetrical bony carotid canal
Fig. 24 3D TOF, hypoplasia of the A2 segment (white arrow) and the contralateral dominant A2 segment “bihemispheric ACA”. Note hypoplasia of the A1 segment (red arrow).
Fig. 25 MIP CTA, right Pcom hypoplasia (arrow). Note the pathological occlusion of the contralateral ICA.
Fig. 26 3D TOF, hypoplasia of the vertebral artery (white arrow) that ends as a PICA (green arrow).
Fig. 27 3D TOF, hyperplasia of the anterior choroidal artery (white arrow). The contralateral anterior choroidal artery is of normal caliber (green arrow). Red arrow points to fetal PCA, blue arrow to Pcom.
Fig. 28 3D MRA, early bifurcation with short prebifurcation segment M1 (arrow).
Fig. 29 Fenestration of the A1 segment (a), Acom (b), M1 segment (c), V4 segment (d) and the proximal part of the basilar artery (e).
How to check the vessels of the neck?
Imaging methods are extremely important for assessing the condition of veins and arteries. For this purpose the following are used:
1. Computed tomography (CT). The method is used to detect acute circulatory disorders and is used for additional diagnostics in case of low information content of MRI. Its advantages are high accuracy and speed, but its disadvantages are radiation exposure and the need to use an iodine-based contrast agent, which creates a number of contraindications to CT.
CT scan of neck and head vessels in 3D modification
2. Digital subtraction angiography. Invasive radiographic procedure. It is not prescribed as a screening because there are faster and safer research methods. Angiography involves the injection of a radiopaque contrast agent into the vascular bed for subsequent assessment of the functions of the arteries and veins in real time. Disadvantages include the need for special training, risk of allergies, radiation exposure, and invasiveness. Advantages: highest accuracy, possibility of simultaneous angioplasty. Angiography should only be performed in the operating room.
3. Duplex scanning. This is a variant of ultrasound examination of blood vessels, which is based on measuring the speed of blood flow. The method is used for emergency diagnosis of obstruction or other pathologies of extracranial (brachiocephalic) vessels and dynamic observation. The advantages are information content, safety, painlessness, lack of preparation and high speed of obtaining results. The disadvantage is the relative accuracy. The method is not very informative at the initial stages of disease development.
4. MR angiography. It does not require special preparation and can be performed with or without contrast. For more accurate visualization of blood vessels, preparations based on a rare earth metal (gadolinium) are used. These compounds are bioinert and safer than iodine-containing products. The advantages of the method are the minimum number of contraindications. MR angiography is safe and painless. The results of the study are quite informative. Native diagnostics is allowed for pregnant women according to indications, starting from the second trimester.
The need for contrast during MRA is determined by the attending physician. Depending on what the MRI of the neck vessels shows and how clear the images are, the radiologist may also order a repeat examination with contrast.
MR angiography is a highly informative procedure that allows you to study not only blood vessels, but also soft tissues of the neck, nerves, and lymphatic structures. The scanning results are layer-by-layer images of the area under study (slices) in three mutually perpendicular projections, with the help of which the localization of pathological changes (tumors, aneurysms, etc.) can be accurately determined.
MRI of head vessels and brain matter
Tomography is based on the phenomenon of nuclear magnetic resonance. The method does not carry radiation exposure and has no restrictions on the frequency of application. The magnetic field is safe for tissues, organs and systems. MR angiography can be used to monitor the dynamics of disease development and evaluate the effectiveness of prescribed treatment.
The disadvantage of MRI is its cost and procedure time. Studying one area in native mode takes 15-20 minutes, and with contrast - 30-35.
1.2. Change in the number of vessels
1.2.1. Reducing the number of vessels
ICA agenesis: 0.01% Fig.11 , Fig.47 :
Around day 24 of embryogenesis, the ICA develops from the dorsal aorta and third arch. Subsequently, at approximately the 5th - 6th week, the base of the skull begins to take its shape. Thus, absence of ICA will result in absence of the carotid canal, identification of which is the most practical method in identifying this anomaly in a clinical setting. Typically, patients with ICA agenesis are asymptomatic due to well-developed collateral circulation through the ECA and vertebrobasilar system [2].
Aplasia of the A1 segment: 1-2%. Fig.12 and Fig.15 :
In this situation, both A2 segments are supplied by the existing A1 segment [2].
Azygos ACA: less than 1% Fig. 13 :
Both A1 segments form a common A2 segment, which supplies blood to both hemispheres[2].
Lack of Acom: 5% Fig.14 [2]:
Typically, the absence of the anterior communicating artery (Acom) is not easy to detect on time-of-flight MR angiography because the artery may be present but the flow signal is too weak to be visualized.
Lack of Pcom: 0.6% Fig.15 :
The posterior communicating artery (Pcom) is usually smaller than the P1 segment. Complete absence is rare [2].
Artery of Percheron: 4-11.5% Fig. 16 :
The thalamo-mesencephalic arterial supply can be divided into 3 types: type 1 is the most common, with perforating arteries on both sides arising from the P1 segments; type 2 , known as the artery of Percheron, arising from one of the P1 segments, supplying both sides; type 3 is an arch that connects both P1 segments and from which the perforating arteries arise [5].
1.2.2. Increase in the number of vessels
Incremental MCA: 2.7% Fig. 17 :
Literary definitions of accessory middle cerebral artery (MCA) and MCA duplication are quite dichotomous. In this paper, we use the definition of Teal et al., who limited the term “accessory MCA” to the branch arising from the anterior cerebral artery (ACA) and the term “duplicate MCA” to the artery arising from the distal segment of the ICA [6]. To distinguish an accessory MCA from a duplex one, the dominant vessel must be identified by carefully searching for the MCA bifurcation. Comparison with the contralateral side is also useful to find the level of ICA bifurcation [1].
Duplication: refers to two separate arteries that do not exhibit distal fusion. For example:
- MCA duplication: 0.2-2.9% Fig. 18 : MCA duplication is an artery that arises from the ICA and runs parallel to the main trunk of the MCA. This variant should not be confused with early branching of the MCA, in which a short single M1 segment is present. It should also not be confused with the anterior temporal branch, which often arises from the M1 segment.
- Acom doubling: 18% Fig. 19 [1].
- SCA doubling: 14% Fig. 12 [2].
Trifurcation:
- ACA trifurcation: 2-13% Fig. 20 : trifurcation refers to the presence of three A2 segments and is described by various names such as pericallosal triplex, arteria mediana corporis callosi and persistent primitive median artery of the corpus callosum [2]. Early origin of a frontopolar branch, for example from Acom, may appear as a third A2 segment.
MCA trifurcation: 12% Fig. 21 : The horizontal segment of the MCA is divided into superior and inferior trunks in approximately 78%. In 12% there is an additional (middle) trunk, this situation is called trifurcation, and the presence of more than 3 trunks, for example, quadrifurcation, is observed in approximately 10% Fig. 22 [2].
Fig. 11 Agenesis of the left ICA. TOF MRA (a), no signal from the flow in the left ICA. MIP CTA (c), CCA continues as ECA with no ICA. Bone window CT (b), absence of bony carotid canal on the left side. The normal carotid duct on the right side is marked with a red arrow for comparison.
Fig. 12 3D MRA, absence of A1 segment of ACA, both A2 segments extend from the contralateral side. Note the partial fetal PCA (white arrow), duplication of the superior cerebellar artery (blue arrow), and hypoplastic vertebral artery (red arrow) that terminates as the PICA.
Fig. 13 3D MRA, fusion of both A1 segments to form a single A2 segment (azygos ACA) (arrow).
Fig.14 3D TOF MRA, no Acom.
Fig. 15 3D TOF, absence of Pcom and A1 segment on one side. The significance of this option is that if the ICA is occluded on that side, there will be no possibility of collateralization through the circle of Willis. Incidental finding: aneurysm of the terminal portion of the contralateral ICA (arrow).
Fig. 16 MIP (a) and 3D TOF (b), type 2 thalamo-mesencephalic arterial supply (artery of Percheron) with a single arterial trunk (arrow) arising from the P1 segment, the branches of which supply blood to both sides.
Fig. 17 3D TOF, additional MCA (arrow) extending from the A1 segment.
Fig. 18 3D TOF of a duplicated MCA (red arrow) extending from the distal ICA. This artery should not be confused with the anterior temporal branch (white arrow), which is a common finding.
Fig. 19 3D TOF, Acom duplication (white arrows), proximal A2 segment fenestration (red arrow), A1 segment aplasia and complete fetal PCA (blue arrow).
Fig. 20 3D TOF, ACA trifurcation with three A2 segments (arrows), the third branch arises from Acom
Fig.21 3D TOF, MCA trifurcation with additional middle trunk.
Fig.22 3D TOF, MCA quadrifurcation.
Indications for MRI of neck vessels
Pathologies of extracranial arteries and veins lead to circulatory disorders in the brain and deterioration of the trophism of its tissues. Such conditions have various manifestations. Indications for MRI of neck vessels are:
- dizziness;
- nausea, morning vomiting;
- fainting;
- sudden attacks of weakness;
- blood pressure surges;
- headache;
- discomfort in the neck;
- speech and swallowing disorders;
- hearing loss;
- blurred vision;
- memory disorders;
- sleep disorders;
- noise in ears;
- poor coordination of movements;
- impaired sensitivity of the skin of the hands, etc.
The method is used in planning operations, as well as for assessing brachiocephalic circulation a certain time after injury. It is necessary to monitor the condition of important vessels in some systemic diseases, for which MRI of the arteries and veins of the neck is prescribed, namely:
- multiple sclerosis;
- diabetes mellitus;
- atherosclerosis;
- vegetative-vascular dystonia;
- hypertension.
Most often, MR angiography is prescribed to clarify the diagnosis when duplex scanning has low information content.
Meaning
The list in Fig. 35 shows the values of the anatomical options.
2.1. Recognition of anatomical patterns and the ability to distinguish them from pathological changes:
2.1.1. Knowledge of normal variations is part of the anatomical knowledge that is important for every radiologist and surgeon. Knowledge of normal variants and their proximity to other structures facilitates the understanding and diagnosis of various diseases, such as:
- Trigeminal neuralgia, which can be caused by the presence of a variant of PTA (less commonly PTA), due to the proximity of the vessel to the trigeminal nerve [11].
- Glossopharyngeal neuralgia or hypoglossal nerve palsy, which can be caused by a persistent hypoglossal artery [1].
- Pulsatile tinnitus in cases of persistent stapedial artery [1].
2.1.2. Option against pathology:
- Infundibulum: The Pcom infundibulum should not be confused with an aneurysm Fig. 36 .
- ICA hypoplasia may be confused with dissection or fibromuscular dysplasia, while ICA agenesis may be confused with occlusion. Visualization of the skull base aids differentiation, as the bony carotid canal will be narrow in cases of hypoplasia and absent in cases of agenesis, but will appear normal in other acquired diseases Fig. 11 and Fig. 23 .
- Different patterns of perfusion abnormalities may occur with normal variations that can cause confusion, especially in the context of stroke:
- Asymmetry of CT or MR perfusion in the occipital lobes, in the case of unilateral fetal PCA. The contralateral side may show delayed perfusion because it is supplied by the posterior circulation Fig. 37 .
- Bilateral perfusion delay in the occipital lobes compared with the frontal and parietal lobes may be observed in the absence of bilateral Pcom Fig. 38 .
- Relative hypoperfusion in the PICA territory in cases of vertebral artery hypoplasia. Hypoperfusion may present as prolonged time-to-peak, prolonged main transit time, or decreased cerebral blood flow, but it never affects cerebral blood volume ) Fig.39 [12].
2.2. Hemodynamic effect of normal variants and abnormalities:
2.2.1. Understanding collateral function: The presence of hypoplasia or aplasia of segment(s) in the circle of Willis can affect collateral function when one or more arteries are occluded Fig. 15 .
2.2.2. Explains unclear cases of stroke:
Vascular conditions that cause changes in unexpected vascular territories can be explained by normal variations such as:
- Ischemia in the posterior territory may accompany ICA pathology due to the presence of fetal PCA Fig. 40 .
- Bilateral ischemia and ischemia in certain areas may draw attention to the presence of pathology in one of the options, such as:
- Bilateral anterior infarction in case of thromboembolism of azygos ACA or dominant bihemispheric ACA Fig. 41 .
- Bilateral mesencephalothalamic infarction with Percheron's artery Fig. 42 .
2.3. Association with vascular and nonvascular congenital anomalies and other diseases:
2.3.1. Association with aneurysms: Changes in vascular anatomy may be a sign of lack of vascular maturity and vulnerability to aneurysm formation. In the work of Lazzaro et al., normal variants of the circle of Willis were more common in cases with ruptured aneurysms than in cases of unruptured aneurysms [13]. Based on a review of the literature, the following variants and abnormalities were associated with aneurysms: Table 4 Fig. 43
- Fenestrations: The incidence of aneurysms (IoA) is approximately 7% of all fenestrations. A defect in the media of the fenestrated segment and turbulent flow at both ends of the fenestration can lead to aneurysm formation. Additionally, in the work of Hudák et al., fenestration was a common finding in patients with unexplained subarachnoid hemorrhage due to a weak arterial wall [1] [2] [14]
- ICA agenesis and hypoplasia: IoA 67% [2].
- A1 segment aplasia: IoA14% [15].
- Azygos ACA: IoA 41%. Due to increased flow from both segments of A1 [2].
- Persistent dorsal ophthalmic artery: IoA 45% [4].
- Persistent primitive olfactory artery: In the work of Uchino et al, 2 intracranial artery aneurysms were found in 14 patients with PPOA (IoA about 14%); one of them is in the hairpin bend (7%) [9].
- PTA: IoA 14% [1].
- Persistent hypoglossal artery: IoA 26% [16].
- Proatlantal intersegmental artery: IoA 10% [1, 2]
- Other variants and anomalies associated with aneurysms whose cases have been reported but not available include infraoptic ACA, superior anterior communicating artery, accessory MCA, MCA aplasia, variant PTA, and asymmetry of the circle of Willis [1, 2].
2.3.2. Association with other vascular anomalies and diseases:
- Fenestration of the vertebral artery is associated with arteriovenous malformation in 7% [6].
- PTA is observed in vascular anomalies such as AVM, carotid-cavernous fistula, and Moyamoya disease in 25% of cases [17].
- Proatlantal intersegmental artery: The incidence of cerebrovascular disorders such as AVM, vein of Galen malformation and aortic arch variants is 59% [18].
- Spontaneous vertebral artery dissection was slightly more common in subjects with hypoplastic vertebral artery than in controls (30.4% vs. 17.4%). It was also found that spontaneous vertebral artery dissection occurs more often with hypoplastic vertebral arteries than with dominant vertebral arteries (68% versus 32%) [19].
2.3.3. Association with other congenital anomalies:
- Azygos ACA may be associated with holoprosencephaly and migration abnormalities Fig. 44 [1].
- ICA hypoplasia is associated with anencephaly and basal telangiectasia [2].
- Fenestration of the vertebral artery may be associated with vertebral fusion [6].
2.3.4. Association with other disorders:
- Pituitary dysfunction and acromegaly in intrasellar “kissing” carotid arteries[2].
- It was found that migraine with aura is more common in patients with an open circle of Willis [20].
2.4. Preoperative planning for cranial surgery, head and neck surgery, and neurointerventional procedures:
The description of normal variations is very important for surgeons and interventional radiologists, as some of these variations must be considered to avoid catastrophic consequences during intervention.
2.4.1. The risk of catastrophic hemorrhage exists in the following cases:
- Transsphenoidal pituitary surgery in cases of PTA or intrasellar “kissing” carotid arteries.
- Middle ear surgery in cases of persistent stapedial artery and aberrant intratympanic ICA.
- Pharyngeal surgeries such as otopharyngeal tumor resection, tonsillectomy, adenoidectomy and palatopharyngoplasty in cases of aberrant lateral pharyngeal artery.
2.4.2. Knowledge of normal variations is important in interventional procedures. This knowledge may help to gain vascular access, such as dominant versus hypoplastic vertebral or variant access, or avoid complications during procedures such as tumor embolization through ECA catheterization in cases of MMA arising from the ophthalmic artery, which can lead to blindness . Fig.45 Fig.50
2.4.3. The presence of persistent carotid-basilar anastomoses should be excluded before certain procedures, such as the Wada test: in this case, injection of amytal can lead to loss of consciousness and apnea [2]
Fig.35 Meaning of normal options
Table 4: Variants associated with aneurysms and their occurrence
Fig. 36 3D TOF MRA, funnel-shaped dilatation with origin of Pcom (white arrow), which should not be confused with an aneurysm. Note the small aneurysm at the origin of the contralateral Pcom (red arrow). Also note the trifurcation of the ACA.
Fig. 37 MR perfusion, TTP map (a) shows slow perfusion in the left occipital lobe; no abnormalities were found in other perfusion parameters. 3D TOF (b), showing fetal PCA on the contralateral side (arrow).
Fig. 38 TTP perfusion map (a) shows a symmetrical delay in the occipital lobes, no deviations in other perfusion parameters were noted. 3D TOF shows no Pcom on both sides.
Fig. 39 (same patient as in Fig. 12) with hypoplasia of the right vertebral artery, which ends as the PICA. TTP map shows perfusion delay in the territory of the right PICA. An examination performed 6 weeks later for other reasons (not shown) showed no pathology in this area.
Fig. 40 MRI of a 56-year-old patient complaining of headache shows dissection of the left ICA (red arrows) with intramural hematoma on T2 (a) and T1FS (b). DWI (c) and ADC (d) show subacute infarction in the territory of the left PCA, which is due to the presence of fetal PCA (TOF not shown).
Fig.41 72-year-old patient with hemiplegia, epilepsy and impaired consciousness. DWI (a) and ADC (b) show bilateral infarcts in the ACA territory. DSA (c), shows proximal occlusion of the azygos ACA (arrow).
Fig. 42 Bilateral acute thalamic infarctions on DWI (a). DSA shows occlusion of the P1 segment of the left PCA (b). Minimal recanalization after intra-arterial thrombolysis (c), with mild opacification of the artery of Percheron (arrows) arising from the left PCA.
Fig. 43 Aneurysms (arrows) associated with abnormalities; a) A1 aplasia with Acom aneurysm. b) Azygos ACA with pericallosal aneurysm. c) fenestration of the basilar artery with proximal basilar aneurysm after coiling.
Fig. 44 Axial (a) and sagittal (b) MRI images of a child with holoprosencephaly, anterior cingulate fusion, and abnormal beak and genu corpus callosum. Note the empty flow of the anterior cerebral artery (arrow), which is single (azygos) and displaced anteriorly.
Fig. 45 55-year-old patient involved in an accident. Initial CT scan (a) shows a fracture of the left temporal bone. She later complained of pulsating tinnitus. DSA (c) with flat panel CT angiography (b and d) was performed. Reconstructed images showed the middle meningeal artery (yellow arrows) arising from the ophthalmic artery (blue arrow) and the traumatic AVM (red arrows) draining into the external jugular vein to form an aneurysm (orange arrow). Knowledge of this option is important when planning therapy.
How is an MRI of the cervical spine done?
At the Magnit diagnostic clinic, scanning is carried out by appointment. During its registration, the patient must inform the medical staff about the presence of any metal structures in the body, namely:
- pins;
- knitting needles;
- endoprostheses;
- middle ear implants, etc.
The presence of products and metals with ferromagnetic properties in the body can affect the quality of the images. Clinic staff must be informed of the specific material the structure is made of. You can obtain this information from the medical institution where the operation was performed. You need to ask for an extract from there and take it with you to the procedure to show the radiologist.
MRA of head and neck vessels
You need to arrive for the MRI 5-10 minutes earlier than the appointed time so that you can fill out the documents without rushing. The study is preceded by a consultation at which the patient is asked about contraindications to the procedure, which are:
- first trimester of pregnancy;
- presence of cardiac pacemakers (pacemakers, defibrillators);
- implantation of an insulin pump;
- the presence of metal stents or hemostatic clips in arteries and veins;
- other ferromagnetic implants.
During the interview and introduction, the X-ray technician explains how an MRI of neck vessels is performed and the rules of behavior in the diagnostic room. Patients with claustrophobia are offered to undergo the procedure on an open tomograph. In case of increased anxiety, it is possible to use sedatives, but only as prescribed by a doctor. For patients with acute pain and neuropsychiatric diseases, MRI is performed under sedation or anesthesia in a hospital setting.
Preparing for the examination involves removing all metal objects. The patient must remove jewelry, glasses, hairpins, and items of clothing with such accessories. Electronic devices (phone, watch, hearing aid) remain outside the tomography room. The procedure for performing MRI of neck vessels is as follows:
- The patient is taken to a special room and placed on the tomograph platform. The conveyor moves so that the neck area is in the center of the device frame.
- The laboratory assistant fixes the position of the person’s head, places cushions for convenience, hands the emergency button and leaves the office.
- An x-ray technician watches the procedure from the next room through glass. He checks the functionality of the speakerphone, reminds the patient to remain still until the end of the study, and turns on the device.
During scanning, the tomograph makes sounds (clicking, humming) that may cause discomfort. At the Magnit diagnostic clinic, patients are offered headphones to listen to pleasant music during the examination. You can get regular earplugs instead. Preparation takes 5-10 minutes, scanning lasts 15-20.
1.4. Changing the course
1.4.1. Aberrant lateral pharyngeal ICA, tortuous ICA, and kissing carotid arteries:
During embryonic development, the ICA is thought to begin to unwind as the dorsal aortic root descends into the thorax, providing a direct pathway for the ICA. Failure in unwinding results in tortuosity of the ICA, which runs close to the midline of the posterior pharyngeal wall, called the aberrant lateral pharyngeal artery [6].
This morphology is more commonly seen in older patients or those with hypertension, but should not be confused with the fetal variant, although both have the same meaning (see below). The incidence of aberrant lateral pharyngeal ICA is approximately 5%, but the exact prevalence of the anomaly is unknown as it cannot be differentiated morphologically from tortuosity. Studies conducted by Ekici et al. showed that the least affected age group with ICA tortuosity was the younger age group [8].
The term "kissing carotid arteries" describes the elongated carotid arteries that meet at the midline; can be observed retropharyngeal or intrasphenoidal / intrasellar Fig. 30 [2].
1.4.2. Persistent primitive olfactory artery: 0,14%
The ACA is derived from the primitive olfactory artery, which regresses to form the recurrent artery of Heubner. Violation of regression leads to preservation of the primitive olfactory artery. This artery has an extreme anterior-inferior course in the A1 segment, which moves along the olfactory tract before the posterosuperior transition to the A2 segment, forming a hairpin-shaped configuration [9].
1.4.3. Persistent embryological anastomosis
+ persistent carotid-vertebrobasilar anastomosis:
During embryonic development, the anterior circulation supplies the hindbrain through several anastomoses, since the posterior circulation is not yet sufficiently developed. After the development of the vertebral arteries, these anastomoses regress. Impaired regression results in abnormal communication between the anterior and posterior circulation in the postnatal period. The most common form of these anastomoses is the fetal type PCA (see variants of origin/origin of vessels). Recognizing the course of these abnormal vessels, as well as the level of entry into the skull, is critical for their differentiation Table 1
- persistent trigeminal artery (PTA): 0.1-0.2% Fig. 31 Fig. 48 : PTA originates from the cavernous segment of the ICA and communicates with the basilar artery. Proximal to the level of the anastomosis, the basilar artery is usually hypoplastic. On the angiogram, when viewed from the side, it has a characteristic “Trident of Neptune” or Tau sign configuration, reminiscent of the Greek letter “Tau” [1] [2]. There are two different classifications Table 2 Fig. 32 and Fig. 33 [10].
- PTA variants (Saltzman III): 0.18-0.76%: Arteries that supply the posterior fossa, arising from the precavernous segment of the ICA and not communicating with the basilar artery [1].
- Persistent auricular artery (otic artery): the rarest carotid-vertebrobasilar anastomosis. The existence of the auricular artery is controversial because it has not been identified in lower animals. It passes from the petrosal segment of the ICA to the basilar system through the internal auditory canal [2].
- Persistent primitive hypoglossal artery (PPHA): 0.03-0.26% Fig. 34 Fig. 49 : This artery runs from the cervical segment of the ICA to the basilar artery through the hypoglossal canal. The vertebral artery is hypoplastic. CT scan of the skull base shows an enlarged bony hypoglossal canal [1, 2].
- Proatlantal intersegmental artery: very rare. Connects the cervical segment of the ICA or external carotid artery (ECA) to the vertebrobasilar system. The artery enters the base of the skull through the foramen magnum, which allows it to be differentiated from the hypoglossal artery. There are two types:
- Type I: joins the vertebral artery above the atlas.
- Type II: enters the vertebral artery through the atlas [1, 2].
+ persistent internal-external carotid anastomosis
- Aberrant intratympanic ICA: very rare. This variant is an anastomosis between the ICA and the ECA, as it is believed to arise from agenesis of the cervical segment of the ICA and the development of an anastomosis between the horizontal (petrosal) segment of the ICA and the enlarged inferior tympanic artery, which is a branch of the ECA. The ICA (or rather the enlarged inferior tympanic artery) in this case has a smaller diameter than the usual ICA, with the absence of the ascending part of the carotid canal as it enters the base of the skull, posterior and parallel to the jugular bulb, which resembles a mass in the hypotympanum; there is also no bone plate between the carotid canal and the tympanic cavity [1].
- Persistent stapedial artery: 0.48%. This anomaly occurs due to the persistence of the anastomosis through the stapedial artery, which is usually present during development between the ECA and ICA. The artery arises from the petrosal segment of the ICA, passes through the obturator foramen, and ends as the MCA in the epidural space of the middle cranial fossa. A CT scan of the skull base may show a small canal near the carotid canal. Foramen spinosum, which contains MMA, will be absent. a persistent stapedial artery may be associated with an aberrant ICA [1, 2].
Table 3 shows the frequency of variants discussed, however, there are other rare variants that cannot be included in a single document. Finally, having a fully developed Circle of Willis can be considered an option since it is present in less than 50% of the population [2]
*NB: Frequency varies between authors depending on the type of study performed (CT, MRI, surgical or post-mortem). Frequency may also vary depending on geographic distribution; Published data may not always be applicable to other populations.
Table 1: Types of persistent carotid-vertebrobasilar anastomoses
Table 3: occurrence of anatomical variants
Fig. 30 Coronal MIP CTA of a patient with a history of hypertension shows elongated carotid arteries reaching the midline (“kissing” carotid arteries).
Fig. 31 3D TOF, lateral view (a) and dorsal view (b), persistent primitive trigeminal artery (red arrow) arising from the cavernous segment of the ICA and communicating with the basilar artery, which is hypoplastic to the level of the anastomosis (white arrow). In lateral view, the anomalous artery with ICA resembles Neptune's trident and the Greek letter "tau".
Fig. 32 MIP CTA of persistent primitive trigeminal artery (red arrow) in two different cases. According to Salas there are 2 types: medial sphenoidal or intrasellar (a), which extends into the sella turcica and perforates the dura mater or dorsum sella (green arrow), as in this case, and lateral petrosal or parasellar (b), in which the vessel goes with the sensory roots of the trigeminal nerve, on the side of the sella turcica.
Fig. 33 3D TOF showing two different cases of persistent primitive trigeminal artery (red arrow). Classification according to Saltzman: Type I (a), in which the PCA supplies the superior part of the basilar artery, including the posterior territory, and type II (b) with the fetal PCA (white arrow).
Fig. 34 3D CE MRA oblique (a) and posterior (b) views showing the persistent primitive hypoglossal artery (red arrow) which arises from the cervical segment of the ICA (green arrow) and continues as the vertebrobasilar artery (blue arrow). ECA is marked with a white arrow.
MRI of neck vessels with contrast
Sometimes native MR angiography turns out to be uninformative. Vessels (even of small diameter) can be visualized more clearly by contrast-enhanced examination. It involves the injection into a vein of a drug that increases the vibrations of hydrogen atoms in water molecules in tissues and makes the latter more visible in photographs.
MR angiography of neck vessels
Contrast is required if abnormal vascular development or tumor changes are suspected. With its help, you can assess the condition of the veins and arteries and the speed of blood flow in them. The procedure for MR angiography with contrast is as follows:
- After standard patient preparation, native scanning begins.
- Once the shooting is complete, a contrast agent is injected into a vein in the forearm.
- Repeat the study.
The procedure takes 30-35 minutes. Contrast MR angiography is contraindicated in pregnant women. During lactation, you need to make a supply of breast milk for the next 2 feedings, which you will have to skip.
Interpretation of photos of MRI images of neck vessels
The scanning results are layer-by-layer monochrome images of the area under study. They are deciphered by a radiologist. The specialist sees and records any deviations in tissue structure from the norm. All pathological changes will be recorded in the conclusion, and the images will be recorded on digital media.
Preparing results takes from 15 to 60 minutes. During this time, the patient can walk or rest in the waiting area. Study protocols are sent by email if you specify it when filling out the documents. You can pick up the original on any other day.
The radiologist describes what MRI shows in the arteries and veins of the neck, he gives explanations when presenting the results and recommendations on which doctor to contact. The specialist does not diagnose, make prognosis or prescribe treatment. If results are received by email, the patient will not be able to consult with a radiologist.