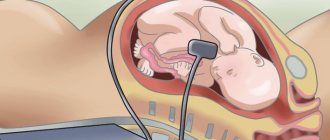
Pathologies in the formation and development of the neural tube begin at 2-4 weeks, when a woman does not even know that she is pregnant. Among the reasons leading to disruption of the formation of the neural tube are external factors: alcohol poisoning, smoking, maternal illness, taking medications, and unhealthy diet.
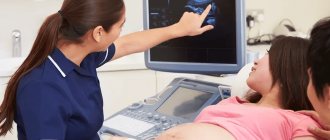
The defects of this organ are so severe that they are often incompatible with life. And it is impossible to detect an anomaly with the naked eye. The pathology of neural tube formation can only be detected by screening ultrasound of the fetus, so this study cannot be ignored.
| Price for screening ultrasound in our clinic | |
| Recording the study on DVD + PHOTO - AS A GIFT! | |
| 1st trimester: Ultrasound during pregnancy with early diagnosis of congenital malformation and calculation of the risk of Down syndrome (11 weeks 6 days – 13 weeks and 6 days), one fetus, using 3D/4D reconstruction | 2000 |
| 1st trimester: combined test with calculation of the risk of Down syndrome + blood sampling for biochemical screening (PAPP-A and free beta-CG) and fetal ultrasound (11 weeks 6 days – 13 weeks and 6 days), one fetus using 3D/4D reconstruction | 3300 |
| 2nd trimester: Ultrasound during pregnancy (18 weeks 0 days – 21 weeks and 6 days), one fetus, using 3D/4D reconstruction | 3100 |
| 3rd trimester: Ultrasound during pregnancy (30 weeks 0 days – 34 weeks and 6 days), one fetus, using 3D/4D reconstruction + Dopplerometry | 3600 |
Anencephaly
The content of the article
Anencephaly is the complete or partial absence of the cerebral hemispheres, and in some cases, the skull bones and soft tissues. Occurs in 1 case out of 10,000, the anomaly is accompanied by other disorders - cleft lip or hard palate, absence of the pituitary gland, spina bifida.
This happens if for some reason the anterior neuropore does not close at 3-4 weeks of pregnancy. Because of this, the frontal extensions of the neural tube, from which the cerebral hemispheres would later begin to develop, do not develop.
Instead of the “gray matter” rich in neurons, fibrous tissue is formed, in which single nerve cells, cystic formations and blood vessels are present. In 71% of cases, the fetus lacks the fronto-occipital zone and the spinal column, in 24% - the occipital lobe with the spinal column, and in 5% - the temporo-parietal zone. The baby's body does not have any abnormalities.
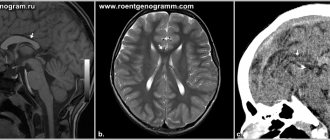
On ultrasound, anencephaly is diagnosed at 11-12 weeks of pregnancy, the accuracy is 96%.
The pathology is characterized by the following echo signs:
- the skull bones are not visualized;
- soft tissues of the brain are anechoic;
- in the vascular system of the brain there is a malformation - an incorrect connection of blood vessels, veins and arteries;
- maternal polyhydramnios (excess amniotic fluid).
The diagnosis of anencephaly is not limited to ultrasound examination alone. When there is a neural tube defect, the hormone alpha-fetoprotein increases in a woman’s blood. Once the diagnosis is confirmed, she is advised to stop the pregnancy, because if the newborn does not have a brain, the lungs, heart, and kidneys will fail after a while.
Ultrasound diagnosis is the primary, but not the main method of diagnosis. If neural tube pathology is detected during an ultrasound examination, the woman is prescribed amniocentesis - taking amniotic fluid from the amniotic sac under the control of a transabdominal sensor for the purpose of laboratory testing.
If a high concentration of alpha-fetoprotein and the enzyme acetylcholinesterase is detected, the pregnant woman is sent for MRI (magnetic resonance imaging). Unlike ultrasound, this method allows you to see the fetal brain in a 4D projection, magnifying the image several times.
The sooner a diagnosis is made, the sooner a woman can terminate her pregnancy. It makes no sense to preserve it with such a diagnosis, because the baby will live for a maximum of a week.
Structure and layers
During the development of the nervous system, three layers appear in the tube:
- ependymal or internal;
- mantle or intermediate;
- marginal veil or outer layer.
The first layer serves as the basis for neurons and gliocytes of the nervous system. Some neurons move into the peripheral part of the organ, forming the mantle layer, and the rest form glial cells.
The most important of these, ependymocytes, line the inside of the tube, which will then develop into the central spinal canal and the walls of the ventricles of the brain.
In the inner layer, separate functional zones are distinguished, which over time transform into one another. The division zone contains cells, among which those closer to the center extend processes to the outer edge and move the body of the neuron containing the nucleus there.
At this time, DNA synthesis occurs in it, after which the particles return to their place. The cells “sitting” on the lower edge are divided into two.
At the migration site, these halves interact. One of them begins to divide again, and the other, using it, moves to the intermediate layer.
The intermediate layer appears thanks to neuroblasts - migrant cells that have not lost the ability to divide and are attached to the inner part of the layer.
Neuroblasts gradually form axons, which are sent to the outer layer, thus forming a dendritic tree and turning into neurons. Unattached cells transform into spongioblasts. From the intermediate layer, such a part of the spinal tissue as gray matter is formed.
The layer on the outside consists of the processes of neurons contained in the mantle layer and blood vessels. It acts as the basis for the development of white matter in the brain.
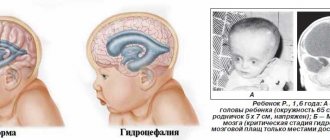
Hydrocephalus
Hydrocephalus is a fetal developmental abnormality in which the number of ventricles in the brain increases. Normally, there should be four of them, and through them the circulation of cerebrospinal fluid occurs. When the production or absorption of cerebrospinal fluid into the circulatory system is disrupted, fluid accumulates in the ventricles, expanding them.
The cause of the pathology is the improper development of the neural tube of the embryo. This happens in the early stages of up to 4 weeks, and in 20% of cases the cause is intrauterine infections.
With hydrocephalus, the accumulated cerebrospinal fluid stretches the ventricles, putting pressure on the brain. As a result, the skull expands and the head takes on an irregular shape.
In the early stages, the defect is difficult to notice; it is detected starting from the 10th week of pregnancy. On ultrasound, the expansion of the frontal lobes is noticeable, the fontanelle becomes more convex, and the head is disproportionately large.
The gynecologist, conducting a screening ultrasound, takes measurements between the temples along the superciliary line (BPR) and, starting from the 16th week, measures the distance between the forehead and the back of the head (LZR). Comparing the results with normal indicators characteristic of a given stage of pregnancy, the doctor makes other measurements.
An increase in the indicators of BPR and LZR does not always indicate pathology. It is quite possible that the baby himself is large if the other measurements of his body also exceed the norm. Only if the head parameters are significantly increased with normal body parameters, the doctor diagnoses “hydrocephalus”.
It is head measurements that make it possible to distinguish hydrocephalus from a congenital increase in the size of the cerebral ventricles. With this disease, there is no intracranial pressure, so the size of the skull is normal.
Hydrocephalus is often accompanied by other developmental defects. Ultrasound shows smoothing of the convolutions of the brain, abnormal formation of blood vessels, anomalies in various parts of the spinal cord, and an increase in the hemispheric fissure.
Another sign of hydrocephalus in the fetus is uterine hypertonicity throughout pregnancy. If the cause of the development of the pathology is an infection, then the woman will be worried about feeling unwell.
The specialist assesses the degree of development of hydrocephalus and gives recommendations and forecasts regarding the baby’s future. In some cases, the abnormality of the neural tube in the fetus is so pronounced that treatment of the child will be ineffective. In this case, the woman is offered an abortion. However, the opposite situation happens, and after treatment the baby will lead a full life.
Congenital diseases and malformations of the central nervous system (CNS) are one of the main causes of perinatal and early childhood mortality, as well as psychoneurological disability from childhood [1, 2]. Mental retardation in 85% of cases is congenital. Each case of the birth of a disabled child represents a serious medical, social and economic problem [3-5]. Its solution requires the most complete information about the features and nature of the central nervous system lesion even before birth.
Different countries around the world have their own approach to the possibilities of regulating the birth rate of children with developmental defects. In recent decades, there has been a trend towards refusal to terminate pregnancy or early delivery for medical reasons, due to ethical, religious or other reasons. In addition, the successes of modern pediatric surgery in some cases allow us to look optimistically at the prognosis in the treatment of a number of diseases that were previously considered unpromising. Malformations of the central nervous system have traditionally been regarded as severe congenital diseases with a poor prognosis, which are accompanied by either the death of the patient or severe disability.
The prevalence of neural tube defects (NTD) in different countries ranges from 1 to 7 per 1000 births. According to French researchers, in Eastern France spina bifida
occurs in 0.62 cases per 1000, anencephaly in 0.33 per 1000 and encephalocele in 0.14 cases per 1000. NTDs are predominantly anomalies of multifactorial etiology. There are known cases of combination of NTD with trisomy and triploidy and some other variants of chromosomal diseases.
Various teratogenic factors (drugs), maternal diseases (diabetes mellitus, hypothyroidism, etc.) are known. Some variants of NTD, which are syndromic in nature, have not yet found an explanation (extraphation of the cloaca in combination with myelocystocele, sacrococcygeal teratoma in combination with meningomyelocele, as well as multiple encephalocele) [6-9]. One of the most well-known causes of birth defects is folate deficiency.
NTDs are common complex multifactorial disorders of neurulation during the formation of the brain and spinal cord in the human embryo on days 21–28 after conception [10]. When NTD is detected in the fetus, in most cases, the course of pregnancy, according to a large number of literature sources, is accompanied by severe polyhydramnios, which manifests itself already in the second trimester of pregnancy. Most publications indicate a more frequent development of polyhydramnios in the case of open spina
bifida
and anencephaly.
Such forms of defects are often accompanied by an increase in the level of α-fetoprotein (AFP) in the amniotic fluid and blood serum of the pregnant woman. Closed forms of spina bifida
and other CNS defects that are not accompanied by liquorrhea often do not lead to changes in normal AFP levels [11, 12].
Of particular interest for study are fetuses with the presence of spina bifida
, since this defect is one of the most common, and the literature contains various information about the outcomes of such pregnancies and the prognosis of the children born [13, 14].
An important difference in the prognosis of a fetus with spina bifida
is the differentiation of the malformation into open and closed forms.
In the presence of an open form of spina bifida,
the prognosis for the fetus is significantly worse than in the case of a closed form. In addition, spina bifida in 70-95% of cases is combined with brain anomalies such as Chiari malformation type II [15, 16].
Antenatal differential diagnosis of open and closed forms of spina
bifida
, according to foreign researchers, is complex.
However, a child born with an open form of spina bifida
demonstrates severe neurological symptoms in the first hours after birth.
Apnea of central origin and other neurological and respiratory dysfunctions are possible. In most cases, motor and sensory disturbances are observed, depending on the level of spina bifida
(80% - lumbosacral localization).
Upon further observation of such children, in addition to the indicated symptoms of dysadaptation, spastic phenomena are observed in the urinary tract and rectum. Infection is often associated. The most common complication is internal hydrocephalus (in 70% of children after birth). According to some researchers, hydrocephalus is the main cause of mental retardation in children with open forms of spina bifida
.
It should be recognized that pregnancy management and general clinical prognosis in children born with spina bifida
have changed over the decades.
In the 60-70s. last century, most reports indicated a poor prognosis and a large number of complications. It was subsequently shown that early surgical treatment of newborns can reduce the incidence of complications such as infection, progressive hydrocephalus, and motor and sensory symptoms. There are isolated reports of antenatal surgical treatment of open forms of spina bifida
, which, according to the authors, can also reduce the number of complications and improve the general and neurological condition of the child after birth.
Delivery by cesarean section is also recommended to reduce the likelihood of mechanical impact on the hernia area and prevent infection. A number of authors indicate that surgical treatment of a newborn with open spina bifida
is only the first step. There is a constant need for corrective and rehabilitation therapy with the involvement of pediatric surgeons, pediatricians, urologists, neurologists, psychotherapists and psychologists.
Despite some successes in the treatment of open forms of spina
bifida
and meningomyelocele, the prognosis in all these cases after birth remains serious. Only isolated observations indicate a favorable outcome.
Cranial hernias are divided into cranial meningoceles and encephaloceles. According to pediatric literature [3, 17], only surgical treatment is possible. Most cases of cranial hernia have an extremely poor prognosis due to the large number of complications and frequent combination with various anomalies and genetic syndromes (Meckel-Gruber, trisomy 13, Goldenhar syndrome, Roberts syndrome, etc.). There is evidence that anterior hernias (frontal and nasofrontal) have a better prognosis compared to occipital and parietal hernias. Anecdotal reports indicate that, despite treatment, about 40% of children with a hernia die. In all cases, severe motor disorders occur, and 50% of surviving children may experience normal intellectual development [16, 18].
Considering the variety of causes leading to malformations in the fetus, it is necessary to use currently known measures that reduce the risk of developing birth defects. By analyzing the various etiological factors of fetal malformations, genetic and non-genetic causative factors can be distinguished (for example, nutritional status or maternal obesity) [19–21].
There are conflicting data in the literature about the role of folates in the development of NTDs. On the one hand, there are reports of normal serum folate levels in mothers whose children were born with NTDs. Studies [22, 23] on cultured embryos of rats or mice with folic acid (FA) deficiency showed the absence of NTDs in its deficiency. On the other hand, according to studies conducted over the past decades [24], it has been noted that the development of NTDs is associated with vitamin deficiency, and exogenous or periconceptional intake of FA into the body of the expectant mother helps reduce the risk of NTDs in the fetus. It should be noted that not only folate deficiency in a pregnant woman’s body is a risk factor for the development of NTDs, but also the specific course of the entire methylation process affects the formation of NTDs [20, 25]. A number of studies [26] report the prevention of NTDs and correction of biosynthesis defects upon the supply of exogenous FA and thymidine in homozygous splotch (Pax3) mouse embryos.
Thus, since FA deficiency may be a risk factor for NTDs, additional studies are needed to evaluate the mechanistic role of FA metabolism in the development of NTDs.
To confirm the influence of FA in the genesis of the development of NTDs, R. Smithells et al. [27, 28] conducted a study using a diet supplemented with multivitamins (FC 0.36 mg/day) in women who had previously had children with NTDs. The control group included pregnant women who did not take vitamins. The results of this multicenter study, published in the 1980s, showed a reduction in the incidence of fetal NTDs in women taking vitamins by 83–91% compared with those in the control group [27–30]. These results indicate for the first time that vitamin or FA supplementation may play an important role in embryogenesis and reduce the incidence of fetal NTDs in repeat pregnancies. Later, in 1991, after a randomized controlled trial (RCT) conducted in 33 centers in 7 countries, the British Medical Research Council stated that in women with a history of fetal NTD, a daily intake of 400 μg of FA is an effective method to reduce the incidence of NTDs in repeat pregnancies by 70% [31]. This was later confirmed by the results of an RCT conducted in Hungary in 1992: the use of FA at a dose of 0.8 mg/day in the periconceptional period led to a significant reduction in the incidence of primary NTDs [32]. In 1991, the CDC recommended FC 4000 mcg/day before and during pregnancy for women with fetal NTDs in a previous pregnancy [33]. In 2000, the medical genetic service of the Moscow Regional Research Institute of Obstetrics and Gynecology developed and introduced into practical healthcare in the Moscow region a program for primary mass prevention of folate-dependent malformations.
By 2005, 75% of pregnant women in the region were taking FC periconceptionally at a daily dose of 0.8 mg. During the period from 2000 to 2005 in the Moscow region, according to the regional register of congenital malformations in children, the proportion of folate-dependent congenital malformations: NTD (anencephaly, spina bifida) decreased from 6.44 in 2000 to 1.91 in 10,000 births in 2005, as evidence of the effect of ongoing primary preventive measures.
Currently, folate prophylaxis is widespread in the Russian Federation, and FA is included in the list of recommended medications for pregnant women during the planning period and the first trimester of pregnancy [34, 35].
In 2007, Canada proposed including obesity (BMI over 35) as a risk factor and recommending a strategy of taking FC in higher doses (5 mg) for patients with a history of low compliance with medications and additional lifestyle factors (alcohol consumption). , smoking, taking medications without a prescription). To prevent fetal NTDs in the presence of epilepsy and diabetes mellitus in the mother, it is recommended to take FA at a dose of 4-5 mg/day [36, 37].
To understand the effect of folate deficiency on the increased risk of developmental defects, it is necessary to recall the features of folate metabolism. FA is essential for one-carbon metabolism, which plays an important role in various cellular reactions. Transmembrane transfer and transport of folate are carried out by receptors and specific transporters [38]. Under normal conditions, dietary folate is absorbed from the intestines, enters the liver and is first converted to tetrahydrofolate and then metabolized to 5-methyl-tetrahydrofolate (5-methyl-THF). FA found in fortified foods and dietary supplements is primarily metabolized to dihydrofolate by the enzyme dihydrofolate reductase in the liver and then converted to tetrahydrofolate (THF), a substrate for polyglutamate synthetase. The polyglutamate form of THF, derived from dietary intake of FA or folate, serves as the primary folate acceptor molecule in the one-carbon cycle. THF is then converted to 5,10-methylene-THF by pyridoxine-dependent serine hydroxymethyltransferase and then irreversibly metabolized to 5-methyl-THF by the enzyme MTHFR (methylenetetrahydrofolate reductase). 5-methyl-THF acts as the primary donor of methyl groups for the remethylation of homocysteine to methionine. Methionine is the main substrate for the synthesis of SAM (S-Adenosylmethionine, S-adenosylmethionine), which plays an important role in methylation reactions catalyzed by DNA methyltransferases with the formation of 5-methylcytosine [39–41]. Thus, the entire metabolism of FA is modulated by several folate coenzymes. The main function of such a coenzyme is to modulate the metabolic pathway by accepting or donating a one-carbon group [41]. The key enzymes in this metabolic pathway involved in the transfer of the methyl group to the homocysteine molecule and the most well studied are MTHFR, methionine synthase reductase (MTRR), reduced folate transporter (RFC), and cobalamin-dependent methionine synthase (MTR) [43]. The etiology of NTDs has long been thought to be related to dysregulation of genes in the major folate pathway or methionine synthase, as well as single nucleotide polymorphisms such as 677C>T in the MTHFR
[44]. Thus, future studies of PK-induced methylation and detailed analysis of the folate metabolic pathway and its role in neural tube closure may provide new information.
The use of dietary supplements, such as FA, and their effects on the genome can have long-term effects on human health without underlying genetic changes. The availability of multiple dietary components involved in one-carbon metabolism, including vitamin B6, choline, betaine, methionine, vitamin B12, and folate, can lead to changes in DNA methylation and histone modification. Modulation of the methylation pattern depends on the content of two metabolites of one-carbon metabolism: SAM (methyl group donor) and SAH (S-Adenosyl-Homocysteine - S-adenosylhomocysteine, an inhibitor of methyltransferases) [45-47]. Thus, nutritional epigenetic factors such as PC (a cofactor in one-carbon metabolism during pregnancy) can influence fetal programming, modulate DNA methylation patterns throughout the genome, and cause dysregulation of gene expression [48]. A study in young children whose mothers took FC in the periconceptional period at a dose of 400 μg showed an improvement in the methylation of the imprinting insulin-like growth factor II gene compared with that in women who did not take FC in the periconceptional period [49]. In addition, several epidemiological and molecular studies have also linked folate intake to epigenetic changes through DNA methylation with neural tube development, including activation of folate receptors (Folr1) and spinal cord regeneration [50, 51]. To understand the FC-induced epigenetic mechanism regulating neural tube closure, a study was conducted on Splotch (Sp–/–) embryos, showing that maternal folate intake during the periconceptional period reduces the number of H3K27 methylation markers and remodels chromatin in the Hes1
and
Neurog2
, genes required for the development of the neural tube [52].
In the literature [27], there is evidence that FA intake changes the expression of some genes, including FMR1
in lymphoblastoma cells. In addition, a study on a mouse model revealed changes in the methylation pattern of the brain epigenome in offspring in cases where the mother received a large amount of FA during pregnancy [53]. Changes in methylation were observed at both CpG and non-CpG sites, resulting in differences in the expression of several key developmental and imprinting genes, and the methylation and expression of some genes varied by sex. The results obtained from the studies indicate that folate plays a key role in the epigenetic regulation of fetal development programming. Therefore, future studies of the role of folate deficiency and intake in epigenetic changes will help to identify the cause-and-effect relationship between FA content and DNA methylation in various diseases.
Nutrition is considered complete if it ensures normal growth, development, disease prevention and resistance to adverse environmental factors. At the same time, adequate nutrition is determined both by the energy value of food and the balance of the diet in proteins, fats, carbohydrates, as well as the provision of vitamins and microelements.
Various conditions of the body - impaired absorption of biologically active substances in the gastrointestinal tract - gastrointestinal tract (gastrointestinal diseases, anorexia of various origins), changes in the metabolism of vitamins (with fermentopathy), a discrepancy between the need for vitamins and their intake from food contribute to the development of hypovitaminosis.
The potential health effects of various fortified foods, dietary supplements including over-the-counter prenatal vitamins, and energy drinks fortified with various vitamins remain important [54, 55].
According to foreign literature [56], a study on mice showed that a 10-fold increase in maternal FA consumption during pregnancy changed the expression of several genes in the frontal cortex of one-day-old pups. Continued consumption of FA at this high dose after withdrawal resulted in behavioral changes compared to those of the offspring of mothers consuming FA at lower doses. These behavioral changes could be caused by changes in gene expression due to aberrant methylation.
Various studies have shown that folate intake can induce aberrant DNA methylation patterns and play a dual role in carcinogenesis. Consumption of F.K. may prevent the appearance of neoplasms at an early stage or potentially cause harm by stimulating the progression of existing preneoplastic formations [57]. A study of children participating in the North Carolina Childhood Leukemia Study found no significant association between folate status at birth and childhood acute myeloid leukemia. The results of RCTs and meta-analysis showed that taking prenatal vitamins containing FA is associated with a significant protective effect in childhood cancers: leukemia, brain tumors, neuroblastoma [58]. Recent environmental studies have found decreased incidence of nephroblastoma, neuroblastoma, primitive neuroectodermal tumors, and ependymoma following dietary FA fortification in the United States and Canada [59, 60].
FA intake during pregnancy and beyond plays an important role in modulating gene expression and disease-related outcomes. Due to the involvement of FA in several cellular processes, including epigenetic modulation and reduction in the incidence of NTDs, the dose, regimen (preconceptional/periconceptional/pregnant) and source of folate during pregnancy and throughout life may be critical. Future clinical and basic research to establish the association between excess folate intake and normal development will help explain the discordance between benefit and possible harm.
According to the literature [61–68], the results of several cohort studies conducted in the USA, Canada, Chile, Australia, several countries in Europe and Asia, revealed the clinical significance of taking FC, although a beneficial effect was not achieved in all cases.
Several countries have introduced mandatory fortification of foods with FA, and although this is effective, no definitive conclusions can be drawn from the data published to date—dosing and potential side effects remain controversial [70]. Due to the fact that FA intake by the expectant mother may induce potential epigenetic effects on the child's genome, with metabolic activity varying individually depending on race, gender, region of residence, and interactions with other nutrients, one possible reason for the discrepancies between studies may be differences in design research. Further international collaboration is needed to accumulate scientific clinical evidence, as well as to interpret the results of intervention studies and potential effects in large cohorts.
According to various authors [71], maternal intake of FA can lead to epigenetic modulation of the child’s brain with general or locally specific changes in genomic DNA methylation, non-coding RNA and histone modification. These effects may alter the expression of some imprinting genes, possible autism susceptibility genes, and major developmental genes.
Over the past 20–25 years, the clinical use of dietary FA supplements for the prevention of NTDs has been observed numerous times. However, given concerns about serum folate levels following the introduction of FA-enriched foods, there is a need to study the effects of FA supplementation on other normal biological processes, such as brain development. Determining the level and distribution of the brain epigenome methylation profile may reveal the mechanism and downstream sequence of various neuropsychiatric and imprinting diseases, including autism. Moreover, since blood folate levels may influence methylation, further studies are needed to examine systemic differences in DNA methylation profiles in relation to the timing of FC intake and dosing in patients of different populations and genders.
Deficiency of vitamins and microelements can have negative consequences in the form of reproductive losses, disturbances in the normal physical and mental development of the fetus. A balanced diet of a woman during the periconceptional period does not fully meet the body’s needs, since during pregnancy, due to the growth and development of the fetus, the body’s need for vitamins and microelements increases significantly. If various complications develop (nausea and toxic vomiting of pregnant women), food consumption is limited and the intake of essential nutrients decreases. Currently, in order to prevent deficiency of FA and other vitamins and minerals, the drug Elevit Pronatal (Bayer), containing 12 vitamins, 4 minerals and 3 microelements, is actively used. This vitamin-mineral complex contains vitamins in the optimal amount for pregnant women: folic acid 0.8 mg, A - 1.2 mg, B1 - 1.6 mg, B2 - 1.8 mg, B6 - 2.6 mg, B12 - 4 mcg, C - 100 mg, D3 - 12.5 mcg, E - 15 mg, biotin - 0.2 mg, calcium pantothenate - 10 mg, nicotinamide - 19 mg. The drug is taken orally after meals, 1 tablet per day 3 months before pregnancy, during pregnancy, after childbirth and during breastfeeding. Elevit Pronatal is recommended for the prevention of FA deficiency. In Hungary, during the implementation of a program to help women during the period of conception using Elevit Pronatal, there were no repeated cases of NTDs in 38 pregnant women who had previously given birth to children with defects.
The effectiveness of Elevit Pronatal was proven in a double-blind, placebo-controlled clinical trial conducted from 1984 to 1991 involving 5,500 pregnant women. The safety of the drug has been confirmed by 15 years of experience in its use in more than a million pregnant women around the world. Elevit Pronatal is the only vitamin and mineral complex with clinically proven effectiveness in the prevention of congenital NTDs. In a study of 5500 pregnant women, the frequency of all types of birth defects in the group taking the drug was 20.6 per 1000 pregnancies, and in the control group it was 40.6 per 1000 [33].
Elevit Pronatal can be used for the prevention of congenital pathology as part of a comprehensive program, including medical genetic examination (karyotyping of spouses, determination of carriage of phenylketonuria and cystic fibrosis mutations, molecular testing of predisposition genes such as the methylene-THF reductase gene, determination of the level of homocysteine in the blood plasma) .
For the prevention of congenital pathology, assessment of health status, correction of deviations in health status and nutritional support before conception for both spouses are very important. The use of vitamin-mineral complexes improves women's health, which contributes to the normal course of pregnancy and the birth of healthy newborns.
Microcephaly
Microcephaly is a complex pathology of the brain, expressed in a decrease in the size of the organ in the fetus. The cause of underdevelopment of the brain is a violation of the division of nerve cells at the stage of formation of the neural tube of the embryo.
The pathology is provoked by several factors: in 40% it develops against the background of cytomegalovirus in the mother; there is also a hereditary form of Giacomini-Penrose-Beck disease.
Microcephaly is rare: 1 case in 5,000, and is often accompanied by other disorders of the central nervous system such as lisencephaly (impaired formation of the cerebral cortex), microgyria (small size of the cerebral convolutions), cerebellar anomaly, and underdevelopment of the spinal cord.
In terms of diagnosis, microcephaly is the most difficult defect. The ultrasound examination is based on the criterion of the ratio of the length of the femur and the circumference of the fetal head, which should not be less than 2.5. However, interpretation is complicated by the fact that the exact gestational age is not always known. False-positive and false-negative results may be due to the small size of the fetus or impaired bone growth in other pathologies.
The accuracy of ultrasound diagnostics for microcephaly is 67.4%, and in 85% of cases the diagnosis is made after 22 weeks of pregnancy. Starting from 2 weeks, the structure of the skull can be easily seen on ultrasound. With microcephaly, it has an irregular shape, the forehead is sloping, the ears are low, and the jaws are underdeveloped. There is also dilatation of the cerebral ventricles.
In addition, in 60% of cases, the fetus is diagnosed with other disorders of the central nervous system, diseases of the kidneys, heart and other internal organs.
Diagnosis of microcephaly is always complex. If a pathology is suspected, a woman's amniotic fluid is analyzed and the fetus is karyotyped. Only after a thorough examination is the woman told about the diagnosis, and she herself decides what to do next.
What is a neural tube?
The neural tube is the basis of the future nervous system that forms in the fetus, which develops from the outer cellular layer (germ layer - ectoderm) along with the skin, while the digestive system is formed from the endoderm.
The laying of the fetal neural tube begins in the third week of its development. First, the neural plate appears, then small elevations called neural folds form at its edges, in the center of which a groove appears. It will later serve as a cavity for the organ. Around day 24, the plate begins to curl and the rollers close, forming a tube shape.
In front it expands, forming the brain vesicles, and the rest will become the spinal cord. Particles of the ganglion plate are gradually separated from the ridges, from which the nodes of the spinal cord and branches of the autonomic part of the nervous system are then formed. You can see the appearance of spinal ganglia in the embryo after 1.5-2 months.
The cells formed in the ganglion plate gradually move to the ganglia in the sympathetic trunk, the adrenal medulla and the walls of the future intestine.
The final formation of the tube occurs at 5-8 weeks, when the development of all fetal organs begins. At this moment, the baby’s heart, lungs, sensory organs, limbs and other systems are formed. The tube itself also becomes more complicated.
Encephalocele
Encephalocele is a type of brain hernia in which the meninges extend beyond the skull through cranial defects. The pathology occurs due to non-separation of the end of the neural tube in the 4th week of pregnancy. As a result, a polypoid mass forms in the fetus: in 75% of cases it is localized on the back of the head or the vault of the skull, and in 25% it protrudes through the facial area.
On ultrasound, encephalocele is diagnosed at the 11-12th week of pregnancy, when ossification of the skull bones occurs. The monitor screen shows a low-echoic formation outside the cranial vault. It consists of medulla, and with meningocele, cerebrospinal fluid is also visible.
In some cases (with encephalocystomeningocele), part of the cerebral ventricle is visualized inside the hernia. In addition, the fetus has microcephaly, hydrocephalus and other intrauterine defects. Typically, with encephalocele, a woman experiences oligohydramnios, which makes diagnosis difficult.
Other signs of fetal encephalocele include:
- wide bridge of nose;
- asymmetrically located eye sockets;
- deformation of the skull.
In early pregnancy, an increase in alpha-fetoprotein is observed.
Ultrasound diagnostics up to 24 weeks of pregnancy is 87% effective, however, with the basal form of the pathology, making a diagnosis will be difficult. In most cases, a woman is recommended to terminate her pregnancy due to severe defects in the child and the impossibility of his rehabilitation in the future.
Among the causes of cerebral hernia are:
- toxic effects on the fetus (smoking, alcohol, drugs);
- taking potent medications;
- infections, mostly hidden;
- viruses (flu, rubella, hepatitis) in the mother;
- work in heavy production;
- genetic factor.
In some cases, if the hernia is small, the baby undergoes surgery during the first 3 years of life. But even with a favorable outcome, he will lag somewhat behind his peers in psycho-emotional development. If the polyp-like growth is not removed, it will attract infections and cause discomfort. In this case, the baby dies within 1 year of life.
Fraction squared
The division of a fertilized egg is called cleavage . This is a process in which the number of cells increases, but their volume remains the same, that is, with each subsequent stage they become denser and smaller. Moreover, this happens quite quickly (one division every 12-20 hours), and by the third day the number of cells in the “lump” or the so-called conceptus can reach 16. By this moment it is already “swimming” to the end of the fallopian tube, in which was located before this (and where, in fact, fertilization occurred) and is ready to enter the uterine cavity.
It is interesting that the crushing itself occurs unevenly, and if you look at the conceptus under a microscope at the end of the second day, you will see that it looks like a conglomerate of tightly welded balls of different sizes.
Attention, a series of new and unusual terms awaits you next, but do not be alarmed - we will explain everything.
The exit of the future embryo into the uterine cavity marks a new stage of its development - the morula (association: for it the cavity is the “sea”). Morula differs from its predecessor in a slightly larger number of cells, as well as their organization: inside the cells are connected by free gap junctions, and outside they form a dense shell - a barrier that isolates the internal environment. And this is not done by chance, because after another day (on the 4th day) a fluid-filled cavity appears in the center - blastocoel .
Morula and blastocyst in the early stages of development
The cell mass is clearly divided into two layers: the outer one - the trophoblast (that very dense membrane), and the inner one - the embryoblast , the cell mass from which organs will begin to develop.
On the 6-7th day, the future embryo “gets tired” of wandering in the “endless” space of the uterine cavity and finds “refuge” on one of the walls, where, with the help of enzymes that lyse (break down) tissue, the trophoblast is introduced and “anchors” the embryo. Now he is ready to develop further.
Prevention. Is it possible to prevent development?
The best way to prevent neural tube defects is to take folic acid, which is a type of vitamin B. Folic acid is taken in the form of dietary supplements or multivitamin complexes. It is also found in some fortified foods, such as cereals, breads, pasta, rice and cereals. Health experts recommend that all women who are pregnant or planning to become pregnant take folic acid. The body's daily requirement is 0.4-0.8 mg.
Doctors also recommend consuming foods rich in folates. In particular, they are contained in:
- dark green leafy vegetables (spinach)
- broccoli
- asparagus
- legumes
- peas
- lentils
- oranges and orange juice.
Causes and risk factors
The reasons for the development of neural tube defects are not entirely clear. They form in the first months of pregnancy, when not all women know about their situation.
The risk of a child developing neural tube defects is higher if:
- Has there been a history of neural tube defects in your family?
- you are obese
- you have diabetes
- you are taking certain anticonvulsants
- you belong to a certain race. Most cases of neural tube defects are recorded among Hispanics.
Other risk factors include genetics and environmental exposures.
Functions
Nervous tissue ensures the normal functioning of all organs and systems. It consists of 2 types of cells:
- neurons are the main structural element; they participate in the reception, processing and transmission of external and internal impulses;
- neuroglia - provide nutrition and protection to neurons, distinguish between groups of neurons with different functions.
Nervous tissue takes part in the formation of the main structures of the brain - central and peripheral.
Reproduction
The lancelet is a dioecious animal. The release of mature eggs and sperm occurs immediately after sunset, fertilization is external (in water). The larvae live in the water column for about three months, feeding on planktonic animals, and then sink to the bottom. The lancelet reaches sexual maturity in the second (third) year of life.
The peculiarities of the embryonic development and structure of the lancelet were studied by the Russian evolutionary zoologist Alexander Onufrievich Kovalevsky (1840-1901), who established the proximity of these animals to the most ancient ancestor of vertebrates.