Brief history, incidence of the disease
James Parkinson is a doctor who first described in detail the symptoms of this serious disease in his work “An Essay on the Shaking Palsy.” The disease was named shaking paralysis on the basis of one of its severe and typical symptoms: constant tremors of the limbs, due to which patients are often unable to fully care for themselves.
Dr. Parkinson described six people who had similar manifestations. Today there are four objective signs that allow a correct diagnosis. Of these, Parkinson accurately described three in the essay:
- tremor (trembling of the limbs) in a calm state;
- hypokinesia (decreased motor activity and limitation of movements);
- postural instability (gradual loss of balance).
A little later, the famous French neuropathologist and psychiatrist Jean-Martin Charcot identified the fourth main diagnostic criterion, adding rigidity (stiffness in muscle movements) to the existing three and naming the disease after James Parkinson.
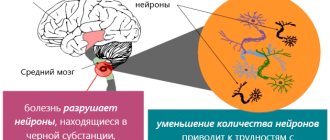
In 1912, Dr. F. Levy described a number of intracellular inclusions in the central nervous system, which are one of the features of the pathology. In 1919, Russian doctor and researcher K.N. Tretyakov found that the main “goal” (or target) of all neurodegenerative changes in PD is the midbrain, in particular, its substantia nigra.
The 60s of the last century became a decisive stage in the search for methods of treating Parkinson's disease. It was then that the drug Levodopa appeared, which experts use to this day.
The disease has a prevalence of 100 - 200 recorded clinical cases per 100,000 people. The main criterion for morbidity is middle or old age (young people rarely get sick). Among patients 60 years of age and older, the incidence rate sometimes reaches 1 to 2%. Some experts believe that PD is more common in males.
Mechanisms of PD development
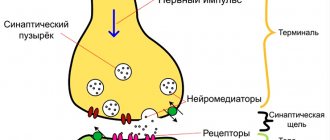
This is a synucleinopathy. When neurodegenerative processes occur, alpha-synuclein protein gradually accumulates inside cells. It is called Lewy body. At the onset of the disease, Lewy bodies can be found only in parts of the peripheral nervous system. They are localized in the plexuses of Meissner and Auerbach, located in the gastrointestinal tract. With further progression of the pathology, Lewy bodies “capture” the nuclei of the brain stem and the substantia nigra of the medulla oblongata.
Severe forms of Parkinson's disease are characterized by the fact that Lewy bodies disrupt the normal functions of the cerebral cortex. In this case, one can judge the topical distribution of neurodegenerative changes: while the substantia nigra is not affected, the clinical picture will be dominated by non-motor symptoms. As the pathology spreads, nigral neurons begin to die, which is accompanied by the appearance of motor symptoms.
Use of extended-release pramipexole in early PD
The development of new dosage forms of antiparkinsonian drugs that provide them with a prolonged release and allow a single dose during the day not only makes treatment more convenient, but also, by improving patient adherence to treatment, increases the long-term effectiveness of therapy. In addition, with a slow release of the drug throughout the day, a more stable concentration in the blood is achieved, which can ensure better tolerability and effective control of disease symptoms throughout the day (both during the day and at night).
A new dosage form of pramipexole with prolonged (controlled) release, which involves a single dose during the day, has been used in European countries and the USA since 2009, and in our country - since 2012. It is a matrix tablet in which the active substance is evenly distributed in a polymer matrix. In the gastrointestinal tract, the matrix absorbs liquid and turns into a gel, which uniformly releases pramipexole over 24 hours. Since pramipexole is highly soluble in a liquid medium, regardless of its pH, the active substance is released from the matrix and absorbed throughout the intestine. The rate of gastric emptying and intestinal motility do not significantly affect the effect of the drug. Absorption parameters also do not depend on whether the drug is taken on an empty stomach or after a meal [2].
When developing a new dosage form, the possibility of a simple, immediate transition from the traditional form of the drug to the new one was taken into account. The condition for this is that equal daily doses of immediate release (taken 3 times a day) and extended release (taken once a day) have the same antiparkinsonian effect. The difference between the new and traditional dosage forms of pramipexole lies only in the rate of release of the active substance. The half-life of pramipexole is the same when using both forms, but controlled release ensures longer maintenance of therapeutic drug concentrations in the blood [5].
The equivalence of equal daily doses of immediate- and extended-release pramipexole has been demonstrated in a number of clinical trials.
It is worth emphasizing the particular convenience of the new dosage form of pramipexole, which can be taken once a day, for patients with early stage PD who continue to work. To avoid side effects, the drug is prescribed by slow titration - according to the same scheme as the immediate-release drug. For this purpose, extended-release pramipexole tablets are available in several strengths: 0.375, 0.75, 1.5, 3 and 4.5 mg. Treatment begins with a dose of 0.375 mg once a day, then, subject to good tolerance, every 7 days they move to the next dose level until the optimal effect is achieved, up to a maximum of 4.5 mg/day (Table 3). After reaching a dose of 1.5 mg/day, it is sometimes advisable to titrate more slowly, since the development of the full therapeutic effect may require several weeks. The recommended dose for maintenance therapy (both in early and in advanced or late stages of the disease) can range from 0.375 to 4.5 mg/day. The most commonly used dose is 3 mg/day.
Table 3. Titration schedule for extended-release pramipexole.
| A week | Dose |
| 1st | 0.375 mg 1 time per day |
| 2nd | 0.75 mg 1 time per day |
| 3rd | 1.5 mg 1 time per day |
| 4th | 2.25 mg 1 time per day |
| 5th | 3 mg 1 time per day |
| 6th | 3.75 mg 1 time per day |
| 7th | 4.5 mg 1 time per day |
Methods of conservative therapy
Motor pathologies manifested in PD are subject to constant drug therapy. There are currently no drugs that can be used as neuroprotectors, so any treatment becomes symptomatic. A number of severe cases of the disease require surgical intervention.
The groups of drugs used in the treatment of PD include:
- levodopa and its derivatives;
- ADR (dopamine antagonists);
- amantadine derivatives;
- MAO-V;
- anticholinergics.
Levodopa and its derivatives are still the main drugs in the treatment of parkinsonism, despite a number of side effects. Long-term therapy is sometimes fraught with complications. In the course of clinical and objective studies, it was found that when using monotherapy with these drugs, treatment is more effective than monotherapy with dopamine antagonists.
The use of levodopa in combination with other drugs against Parkinson's disease has become widespread practice. This tactic allows you to constantly monitor symptoms and make the necessary adjustments to therapy in a timely manner.
Levodopa is characterized by the shortest half-life of elimination from the body. For this reason, at the beginning of the disease it is always prescribed three times a day, carefully increasing the dose as the disease progresses. The mechanism of action of the drug is that it compensates for the deficiency of dopamine in the human body that occurs as a result of the death of a large number of nigostriatal nerve cells.
Dopamine is a substance that cannot pass the BBB on its own. Chemically, levodopa is a precursor to dopamine. After it enters the blood, its gradual transformation into dopamine begins. In pharmacological practice, this process is called decarboxylation.
To increase the concentration of levodopa in the brain when taking it, carbidopa or bensarazide is also used. Because it has different metabolic properties, sometimes co-administration with tolcapone or encapone increases its bioavailability.
In our country, the following combination of drugs is often used:
- levodopa;
- entkapon;
- carbidopa.
There are several dosages of levodopa. It is known that standard dosage forms of the drug are most often used, but sometimes they use a type of levodopa, which is not eliminated from the body so quickly and lasts much longer. This drug is suitable for patients with severe motor impairment.
In addition to levodopa, ADRs (or dopamine antagonists) are used in the treatment of Parkinson's disease. They act on D-2-dopamine receptors and are used together with levodopa and other drugs. Since the disease sometimes occurs in young patients, these treatment regimens are most preferable for them. Modern drugs in this group include a number of non-ergoline agonists:
- pramipexole;
- ropinirole;
- piribedil.
These medications are intended for internal use. There is also rotigotine, available in the form of an adhesive plaster. It is well suited for people who, for one reason or another, are intolerant to oral medications. The drug apomorphine, intended for injection under the patient’s skin, will soon appear on the pharmacological market.
ADRs can have a number of undesirable side effects such as nausea, vomiting, decreased blood pressure, and swelling of the extremities. In severe cases, mental disorders may occur, ranging from severe daytime sleepiness to hallucinatory phenomena.
The motor symptoms of Parkinson's disease, together with levodopa drugs, are corrected with amantadine derivatives (sulfates or chlorides). Amantadine demonstrates a mild therapeutic effect, so it is always recommended to prescribe it together with other groups of drugs. Its function is that it releases dopamine, which the body lacks, and prevents its reuptake. There is a form of amantadine intended for emergency cases: in the form of intravenous injections, when the patient experiences a severe exacerbation with severe dysphagic phenomena.
Free consultation on training issues
Our consultants are always ready to tell you about all the details!
Amantadine group drugs have few side effects. In some cases, nausea, thirst and mild swelling of the extremities may appear. There are also contraindications to its use: the drug should not be prescribed to patients suffering from dementia and hallucinatory syndrome.
MAO-B, or monoamine oxidase inhibitors, increase the concentration of dopamine in the body. These drugs include:
- rasagiline;
- selegiline.
They are used when the disease is just beginning and its symptoms are not clearly expressed. If we compare the effect of MAO-B with other drugs prescribed for Parkinson's disease, their effect is much less pronounced, so they should be prescribed as additional drugs. Recently, rasagiline is most often prescribed, as it has minimal side effects.
Among anticholinergics, patients with PD are prescribed drugs that reduce limb tremor and are resistant to other types of therapy. Typically, doctors use trihexynyphenidyl or biperiden. The substances contained in them can cause side effects: urinary retention, disorders of thinking and perception. Contraindications include glaucoma, dementia and any mental disorders accompanied by hallucinatory phenomena.
Diagnostics
The diagnosis of PD is carried out in 2 stages. At the first (syndromic) stage, parkinsonism syndrome must be distinguished from other conditions that mimic it (Table 1).
Table 1. Conditions requiring differential diagnosis with parkinsonism.
| If there is tremor | In the absence of tremor |
| Increased physiological tremor Essential tremor Dystonic tremor Hepatolenticular degeneration | Apathetic-abulic syndrome Depression Frontal dysbasia Humeral periarthropathy Hypothyroidism Cervical osteochondrosis Dementia with the phenomenon of paratonia (continuity) Catatonia |
Identifying signs of hypokinesia is key in differential diagnosis. Initial symptoms of hypokinesia may be characterized by difficulty writing, pressing buttons on a remote control, brushing teeth, typing on a keyboard, removing small objects such as coins from a bag or pocket, putting on slippers, etc. Sometimes, already at an early stage, weakness and lag of one of the legs when walking appears with a change in the usual gait pattern. Characterized by a weakening of friendly arm movements when walking (acheirokinesis), impaired recharging of the watch (“Rolex symptom”). A weakened voice, slowness, weakening of intonation, or unclear speech may be noticed (especially when pronouncing morphologically complex words quickly). When examined to identify hypokinesia, the patient is asked to perform certain movements for about 20 seconds at the fastest pace and with maximum amplitude. In this case, the doctor should pay attention to the slow initiation of movement, asymmetry of movements, but most importantly - to a special form of exhaustion of movements (decrement), which, as they are repeated, become increasingly slower, decrease in amplitude, and require more and more effort from the patient. The phenomenon of exhaustion can be detected in all movements assessed, but is sometimes noted only in one of the tests. It should be borne in mind that the slowness and awkwardness of movements characteristic of patients with parkinsonism at an early stage can be confused with manifestations of pyramidal and cerebellar insufficiency, as well as severe depression, however, these conditions are not characterized by a decrement of movements as they are repeated. It should be borne in mind that hypokinesia can be difficult to detect against the background of severe tremor in a limb, however, even in this case it is important not to miss a diagnostically significant phenomenon: with parkinsonism, after performing a test for hypokinesia, the patient often holds his hand in a fixed, tense position and is not able to quickly relax.
Muscle rigidity is manifested by stable (as opposed to spasticity) resistance to passive movements in the wrist, elbow, shoulder, knee joints, as well as in the neck, and subjectively by stiffness and unpleasant painful sensations in the limbs. In some patients, when checking their tone, the “gear wheel” phenomenon is revealed. Rigidity should be distinguished from the phenomenon of resistance (gegenhalten), characteristic of patients with dementia and damage to the frontal lobes. Counter-containment changes rapidly depending on the direction and speed of passive movement.
Slow (3–4 Hz) resting tremor in one arm or leg is one of the common initial manifestations of parkinsonism. The presence of a classic resting tremor of the “rolling pills” or “counting coins” type is most characteristic of PD. To identify latent tremor, the patient is asked to make movements with the other hand, walk around, and perform a distraction task (for example, subtracting from 100 by 7). To identify tremors in the leg, you need to examine the patient in a sitting or lying position. However, in the absence of hypokinesia, rest tremor does not allow diagnosing either parkinsonism or PD. It should be taken into account that, on the one hand, essential and dystonic tremor can be observed at rest, on the other hand, postural and kinetic tremor is often observed in PD.
The initial manifestation of PD, especially in young people, may be foot dystonia, which appears or worsens when walking, and much less often - dystonia of other localization.
Early non-motor disorders. Starting from the earliest (prodromal) stage of the disease, the patient may be bothered by emotional depression, increased irritability, fatigue or a feeling of constant fatigue, as well as autonomic disorders such as sweating disorders (“defective thermostat”), for example, profuse sweating in cold weather, and also a tendency to constipation, frequent and/or imperative urination, increased drooling at night (the “wet pillow” symptom), erectile dysfunction. Hypoosmia often occurs already in the premotor stage of PD, but rarely attracts the attention of the patient himself, and a formalized study is necessary to identify it (using special techniques, for example, the University of Pennsylvania Olfactory Test - UPSIT). Identifying signs of rapid eye movement sleep behavior disorder syndrome (anxious dreams, vocalizations, sleep-talking, movements reflecting the content of dreams), which may precede other manifestations of the disease for many years, may be of important diagnostic importance. These non-motor manifestations may improve the accuracy of diagnosis based on early motor symptoms of the disease.
The debut manifestations of PD are also chronic pain syndromes, most often in the back and scapulohumeral region, associated with increased muscle tone, limited mobility and postural disorders.
Already at an early stage, signs of mild cognitive impairment may be detected, in particular instability of attention and slowness of thinking, difficulty finding words (the “tip of the tongue” phenomenon).
"Red flags". The second stage - the stage of nosological diagnosis - comes down to the differential diagnosis of PD with other nosological forms of parkinsonism. It requires a clinical assessment of the history and neurological examination findings. It is important to clarify the drug history. Drugs such as metoclopramide, sodium valproate, cinnarizine, amiodarone can cause drug-induced parkinsonism. Discontinuation of the drug that triggered the development of parkinsonism may not lead to immediate regression of symptoms. Sometimes, after taking the drug and a short-term improvement, the condition worsens again, which indicates a latently developing degenerative process that was “unmasked” by the side effects of the drugs.
A neurological examination may reveal symptoms that are atypical for PD, requiring the exclusion of other diseases that cause parkinsonism. Among them are: symmetry, rapid progression of symptoms with early loss of the ability to move within 5 years, early development of postural instability with falls, lack of a persistent positive effect of adequate doses of levodopa, early development of autonomic failure, rapid onset of dementia (within 1- year), limited mobility of the eyeballs (especially paresis of downward gaze), early development of severe pseudobulbar syndromes, axial dystonia, pyramidal and cerebellar signs, the presence of focal disorders of cortical functions.
Rules for prescribing and side effects of antiparkinsonian drugs
Since we are talking about specific drugs and their combinations, it is important to consider several points:
- clinical course and severity of symptoms;
- the degree of effectiveness of each drug when taken in a particular patient;
- presence or absence of side effects.
If Parkinson's disease began at a relatively young age (before age 50), the first drug to consider is a dopamine receptor antagonist. With late development of the disease (aged 70 years and older), preference should be given to levodopa drugs.
Of course, levodopa should be considered as a kind of “gold standard”, but, unfortunately, it always gives a number of complications in the form of fluctuations in its effect and motor disorders. At first, the patient feels how the effect of the drug ends, and later he experiences “on-off” phenomena. When motor fluctuations appear, patients may experience side effects from the mental, autonomic and sensory spheres. One should also take into account the phenomenon of so-called drug dyskinesia, when a person experiences a series of strange violent movements that he cannot control. As a rule, this phenomenon occurs in the later stages of the disease and marks the peak of the drug's effect.
For severe complications associated with the use of levodopa, it is recommended to resort to methods that stimulate dopamine receptors in the body. We are talking about the introduction of a levodopa-based gel through a gastrostomy tube. Subcutaneous administration of apomorphine is also allowed.
If you have to resort to intraduodenal administration of the drug, you need to periodically show the patient to the surgeon to avoid typical complications. Subcutaneous administration of apomorphine is a more gentle treatment option, although there are side effects in this case.
Additional research methods
At the moment, there are no laboratory or instrumental research methods that would be mandatory for every patient with suspected PD. In recent years, patients with PD have often undergone CT or MRI of the brain, but most often this is not necessary, and in most cases the diagnosis can be made based on clinical findings. However, if the clinical picture in a patient with parkinsonism syndrome deviates from the classic variant characteristic of PD, in particular, there is no typical response to dopaminergic drugs, then neuroimaging is necessary.
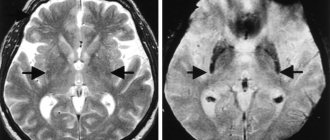
When the onset of the disease is before 50 years of age, it is important to exclude hepatolenticular degeneration, which may be indicated by a corneal Kayser-Fleischer ring, low ceruloplasmin levels, increased signal intensity from the basal ganglia and cerebellum on T2-weighted MRI images, and increased urinary copper excretion.
Transcranial sonography of the deep structures of the brain may also have diagnostic significance, revealing in PD hyperechoic changes in the projection of the substantia nigra, associated with the accumulation of iron and established in 92% of cases of clinically probable PD, but its results can only be interpreted in a clinical context.
Of the practically important, but not yet available in our country, diagnostic methods include positron emission tomography (PET) and single-photon emission computed tomography (SPECT), which make it possible to study synaptic transmission at all levels, as well as monitor the pathological process. When detecting a decrease in the accumulation of F18-fluorodopa with PET and β-CIT with SPECT in the striatum, we can talk about the involvement of presynaptic nigrostriatal terminals in the pathological process (primary parkinsonism). Determination of decreased accumulation of 11C-raclopride (D2 receptor ligand) in PET will indicate a decrease in the number of dopamine receptors in the striatum (parkinsonism “plus”).
Surgical treatment used for PD
In the treatment of PD, the use of neurosurgical methods cannot be ruled out. They are appropriate in the following cases:
- if the medications used do not provide improvement;
- if the tremor at rest does not stop even when taking potent drugs;
- if therapy is effective, but other serious diseases or side effects of medications interfere with its implementation.
For neurosurgical interventions in the case of Parkinson's disease, two methods are used:
- destruction (with penetration into certain parts of the brain, in particular the thalamus);
- stimulation. In this case, the surgeon performs deep stimulation of the patient’s brain, without the use of destruction.
Any destructive operation with such interventions is carried out unilaterally - due to the high risk of postoperative complications. One of the typical complications in this case is pseudobulbar syndrome, characterized by central muscle paralysis. If this happens, the patient has difficulty speaking, swallowing and chewing functions are impaired. Typically, the risk of such events occurring is about 30%.
During stimulating surgical interventions, three areas of the brain are chosen as a “target”:
- two nuclei of the thalamus (subthalamic and ventrolateral);
- area of the globus pallidus.
Stimulation of the ventrolateral nucleus is carried out if the patient suffers from constant tremor of the limbs and cannot care for himself. The internal portion of the globus pallidus in the brain is stimulated when a patient has drug-induced dyskinesia.
The process of selecting candidates for surgery must be thorough and serious. One of the main conditions for the intervention is an established diagnosis of Parkinson’s disease, which has lasted at least five years. You also need to first understand how effective the levodopa treatment turned out to be, since the effect of the operation, if it is successfully completed, will be approximately the same.
Of course, before performing surgery, it is necessary to make sure that the patient does not suffer from serious mental disorders and dementia.
The risk of postoperative complications is usually low, and if patients are selected correctly, they should not give up the chance to improve their condition.
General principles for starting treatment
Since at the moment the ability to slow down the process of degeneration due to the neuroprotective effect (the ability to protect intact cells from damage) or the neuroreparative effect (the ability to restore the activity of partially damaged cells) has not been convincingly proven in any of the drugs used, treatment is still based on symptomatic action. However, the potential for a neuroprotective effect, supported by experimental or clinical data, should be taken into account when prescribing treatment.
Currently, a widely accepted concept emphasizes the importance of early administration of dopaminergic therapy - immediately after diagnosis - in order to more quickly correct neurochemical imbalances in the brain and support compensation processes.
If previously the need for maintaining monotherapy as long as possible was emphasized, at present the advantages of this approach do not seem obvious - compared with an early transition to a combination of drugs with different mechanisms of action. The need for monotherapy or combination therapy should be decided individually. In any case, when choosing drugs and their dosage, one should strive not for complete elimination of symptoms, but for a significant improvement in function, allowing one to maintain everyday and professional activity. At the same time, you should avoid making several changes to the treatment regimen at once (for example, increasing the dose of several drugs at once or adding several drugs at once), this allows you to separately evaluate the effectiveness and safety of each of the prescribed drugs.