Index of the central parts of the lateral ventricles
The index of the central sections of the lateral ventricles is calculated by the ratio of the smallest distance between their outer walls in the area of the recess to the maximum internal diameter of the skull on the same section, multiplied by 100. Normally, the value of this index ranges from 18.2 to 26.0 [1].
Index of the central parts of the lateral ventricles ICVBI = C/Dx100 [1]
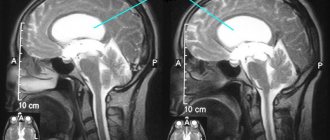
Fig.1 Measurement of the lateral ventricle index
Possible consequences and complications
Probably due to the wide availability of information and the opportunity to consult with other parents, an unhealthy trend has been observed recently. Parents refuse to treat their children for hydrocephalus; they attribute constant crying to capriciousness and stubbornness, and lethargy to character traits. People are frightened by serious medications and contraindications, and they decide that the disease will go away on its own.
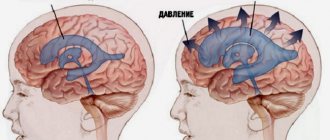
But asymmetry of the ventricles of the brain, their significant increase can lead to serious consequences:
- Delayed mental, physical, mental development.
- Loss of vision: complete or partial.
- Hearing loss.
- Paralysis of limbs, complete immobilization.
- Pathological head growth.
- Inability to regulate bowel movements and urination.
- Epileptic seizures.
- Frequent loss of consciousness.
- Comatose state.
- Lethal outcome.
It’s good if the doctor notes a slight deviation from the norm during the ultrasound and suggests only observing the patient. This is possible if there are no symptoms of the disease: the child is calm, eats well, sleeps, and develops normally.
A diagnosis of “Dilation of the lateral ventricles of the brain in a child” has been made, but you doubt the professionalism of the doctor, and do not want to give your newborn medications in vain? Contact several independent specialists and get a complete examination. Do not refuse treatment, since the actions of the parents determine how fulfilling the child’s life will be.
III ventricle index
The index of the third ventricle is assessed by the ratio of its maximum width in the posterior third at the level of the pineal body to the largest transverse diameter of the skull on the same section, multiplied by 100. Normally, the value of this index increases with age, being equal to 3.0 at the age of up to 5 years and 4 .8 – from 71 to 80 years [1].
III ventricle index = E/Fx100 [1]
The Schlatenbrandt-Nurenberger index (third ventricle index) is the ratio of the maximum transverse diameter of the skull (F) and the width of the third ventricle (E) [2]. A value from 30 to 50 is considered a normal value; a value less than 20 indicates a mild degree of hydrocephalus [2].
Treatment
When a child has enlarged ventricles of the brain, only a neurologist or neurosurgeon can prescribe the necessary treatment.
Drug therapy
Enlargement or asymmetry of ventricular structures does not always require treatment. If the child develops correctly, eats and sleeps well, it is considered that enlargement of the ventricular horns is an acceptable deviation from the norm.
If pronounced neurological symptoms occur, the baby is prescribed special medications:
- Diuretics (Diacarb, Furosemide) - to reduce cerebral edema, speed up urination, and normalize fluid excretion from the body.
- Potassium preparations (Panangin, Asparkam) - to replenish potassium deficiency, which occurs during accelerated work of the urinary tract.
- Vitamins (Multitabs, B6, D3, Magne B6) - to prevent rickets and accelerate regeneration processes in the body of a newborn.
- Nootropic drugs (Cavinton, Vinpocetine, Noofen, Ecephabol, Cerebrolysin) - to normalize cerebral circulation, strengthen blood vessels, improve microcirculation in brain tissue.
- Sedative medications (Glycine) – help reduce nervous symptoms: tearfulness, moodiness, irritability; stabilize the process of falling asleep, normalize sleep.
If the provoking factors that caused the pathological growth of the ventricles of the brain are identified, they are also eliminated: viral and infectious diseases are treated. If the cause of the pathology is brain damage or the growth of a tumor, surgical intervention is performed: the cyst is excised, the cancerous tumor is removed.
When enlarged ventricles of the brain are diagnosed in a child, treatment takes a long period of time. Newborns need to undergo massage courses and constantly perform physical therapy exercises to restore muscle tone and prevent atrophy.
IV ventricle index
The index of the fourth ventricle is calculated by the ratio of its greatest width to the maximum internal diameter of the posterior fossa of the skull on the same section. Normal values for this index are 11.9-14.0. The size of the ventricular system does not depend on gender [1].
IV ventricle index = H/Ix100 [1]
Fig.2 Measurement of indices of the third and fourth ventricles
The Akimov-Komissarenko index is usually used for moderate or slight deformation of the ventricles of the brain. It is determined by calculating the total cranioventricular coefficient using the formula
4d/(a+b+c+c1), where
- d is the transverse diameter of the skull,
- a - the distance between the outer walls of the anterior horns of the lateral ventricles,
- b – width of the anterior sections of the anterior horns,
- c — distance from the superior internal angle to the outer wall of the right lateral ventricle,
- c1 — the same distance of the left ventricle.
A value of more than 5.2 is normal, from 5.2 to 4.8 indicates an insignificant degree of hydrocephalus, from 4.8 to 4.4 - moderate and less than 4.4 - severe hydrocephalus [2].
The corpus callosum (CC) is the largest cerebral commissure, located along the longitudinal midline of the brain. It connects the phylogenetically youngest areas of the cerebral hemispheres, providing interhemispheric information transfer [1].
Agenesis of the corpus callosum (ACC) is the absence of crossing of the midline by commissural fibers of the MC. According to the classification of congenital malformations of the brain and skull by D. Harwood-Nash [2], AMT belongs to the group of disorders of organogenesis and ranks 10th among all other malformations of the central nervous system. The incidence of AMT is 0.3–0.7% in the general population and 2–3% among people with mental retardation [3].
In combination with AMT, developmental defects such as MT lipomas, polymicrogyria, schizencephaly, interhemispheric cysts, Dandy-Walker malformation, etc. can occur. As a rule, AMT does not have an independent clinical picture, which makes its diagnosis difficult [4]. Intravital imaging of AMT has become possible thanks to the introduction of magnetic resonance imaging (MRI) of the brain into clinical practice.
The mechanisms of MT formation still remain the subject of debate. It is known that the nervous system develops from the medullary tube. At the 4th week of gestation, three primary brain vesicles are formed, at the 6-7th week - five. The MT is formed from the first medullary vesicle and is formed from the integumentary part of the lamina terminalis of His, the dorsal end of which thickens, forming the so-called transverse shaft [5]. According to the results of a number of studies [5–7], at the 15–17th week of intrauterine development, commissural fibers of the MT appear, connecting the cerebral hemispheres. According to G. Davilla-Gutierrez [8], first the MT increases rostrally, then caudally, while the fibers of the rostral part intersect approximately on the 74th day, and on the 115th day it thickens. L. Richards et al. [7], on the contrary, indicate that some of the caudal MT axons, dependent on the hippocampus, can simultaneously intersect with the rostral MT axons. By the 20th week, the MT is almost formed [9]. In premature infants, its formation slows down [10]. In infants, by the 8th–10th month of life, final myelination of the rostral part of the MT occurs [11].
AMT may be the result of insufficient development of the commissural plate, agenesis, or destruction of the third layer of neurons [12]. There is evidence that primary AMT is formed before the 12-16th week of intrauterine development [13] .
After 18-20 weeks, secondary damage to the MT occurs, which is a consequence of encephalomalacia at later stages of fetal development [14]. With secondary AMT, underdevelopment of the posterior part (ridge) of the MT often occurs, and the anterior or middle parts of the MT (knee, beak and part of the body) are absent [12].
Certain difficulties in diagnosing AMT continue to this day. The time of its intrauterine detection directly depends on the stage of MT ontogenesis.
According to R. Achiron et al. [15], who examined 270 pregnant women between the 16th and 37th weeks of gestation, during ultrasound examination of the fetus they were able to measure the length, width and thickness of the MT in 258 cases. This made it possible to identify a linear relationship between the increase in body weight and the gestational age of the fetus, and also to establish that its maximum increase occurs at the 19th–21st weeks of gestation. A. Barkovich et al. [16] suggested that certain subtypes of AMT depend on gender. This was confirmed in animal experiments: in laboratory animals (mice) of the BALB/cCF line with disorders of MT development, a predominance of females was revealed [17].
According to studies by P. Govaert and L. de Vries [11], after the 20th week of pregnancy, ultrasound reveals the following signs of AMT: ventriculomegaly; high location of the third ventricle; absence of a cavity of the transparent septum, and according to S.M. Vojvodina [18], on fetal ultrasound, provided that the sagittal projection is obtained technically, AMT is detected at 15–36 weeks of pregnancy. Unfortunately, a significant proportion of cases of isolated AMT are not detected by routine prenatal ultrasound.
Prenatal MRI is most effective in diagnosing AMT [11]. In 74% of cases in patients with AMT, MRI results coincide with ultrasound and CT diagnostic data [19].
The postnatal ultrasound picture of AMT is characterized by the absence of an image of MT; the disappearance of the normal architectonics of the sulci and gyri in the sagittal scanning plane; fan-shaped extension of the grooves from the roof of the third ventricle; wide arrangement of the lateral ventricles with a change in the orientation of the anterior horns in the coronary planes and upward displacement or expansion of the third ventricle. On K.T. a parallel course and an increase in the distance between the bodies of the lateral ventricles, expansion of the posterior horns and vestibules of the lateral ventricles (colpocephaly) are determined [11].
This publication presents our own observations of AMT and analysis of the corresponding data from intravital neuroimaging of the brain.
Our observations show that MRI has an advantage over other methods in identifying congenital malformations of MT, as well as differentiating concomitant anomalies of the central nervous system (Fig. 1, a, b, c, and Fig. 2, a, b, c). From the presented data it is clear that on an MRI of the brain in a full-term newborn, the knee, splenium (thickness about 4 mm), and trunk (body) (thickness up to 2 mm) M.T. are visualized. At the 2-3rd month of the baby’s life, the knee and beak thicken, at the 5-8th months - the bolster. By the 3rd month of a child’s life, due to ongoing myelination processes, the image of the MT cushion on T1-weighted tomograms becomes hyper-intense, and on T2-weighted tomograms it becomes hypointense [11, 12]. The optimal slice for MT imaging is the midsagittal slice [20, 21] (Fig. 3).
Rice. 1. CT and MRI of the brain (sagittal, frontal projections) and appearance of patient T., 8 months. a — CT: AMT (arrow); b — microphthalmos on the left on MRI (arrow); c — appearance of a patient with multiple stigmata of dysembryogenesis.
Rice. 2. Results of examination of patient S., 8 years old. a, b, c — MRI of the brain (sagittal and axial projections): a combination of AMT and diffuse pachygyria (arrows).
Rice. 3. MRI of the brain (sagittal projection) patient S., 7 years old. AMT (arrow).
An important finding in AMT on MRI is the parallel orientation of the bodies of the lateral ventricles in relation to each other (Fig. 4, a), dilated frontal sections of the ventricles, the so-called “grasp symptom”.
Rice. 4. MRI of the brain of patient K., 4 years old. AMT. Axial projection: a - abnormal parallel orientation of the bodies of the lateral ventricles in relation to each other (arrow); b — frontal section: deformation of the anterior and posterior horns of the lateral ventricles, a peculiar U-shaped character of the frontal sections of the lateral ventricles (arrow).
During MRI, we also paid attention to the isolated expansion of the dorsal horns described in the literature [12], colpocephaly, caused by hypoplasia of the associative tracts of the white matter of the occipital lobes. The diagnostic range on MRI is complemented by the absence of normally formed pericallosal gyri (Fig. 5, a) and the radial centripetal position of the grooves on the medial surface of the parietal lobe (Fig. 5, b).
Rice. 5. MRI of the brain of patient A., 3 years old. AMT. a — sagittal projection. Absence of normally formed pericallosal gyri (arrow), b - high position of the third ventricle, radial centripetal direction of the grooves of the medial surface of the parietal lobes.
A new neuroimaging technology has also appeared - magnetic resonance morphometry, which helps to assess the degree of damage to M.T. It is possible to calculate the callosal index - the ratio of the area of the MT to the area of the internal section of the cranium, to estimate the size, total area, thickness of the knee, body, isthmus and ridge of the MT [22-24].
Often, changes in the structure of MT are discovered accidentally during CT or MRI studies for other diseases. Thus, the presence of AMT in a patient with juvenile Huntington’s disease in combination with multiple connective tissue dysplasias and osteochondropathy was described [25].
Thus, among multiple congenital malformations of the central nervous system, which affect about 1% of children [26], one of the leading places belongs to AMT. Currently, neuroimaging methods are being improved, such as fetal MRI, and ultrasound scanning techniques are being used to scan the fetus in the prenatal period in the early stages of gestational development, which makes it possible to suspect a particular hereditary syndrome in the prenatal period.
Adequate and timely recognition of congenital malformations of MT is a difficult task, the solution of which requires knowledge of the latest neuroimaging diagnostic techniques. This article presents the main neuroimaging manifestations of AMT.
There is no conflict of interest.
Dimensions of the III and IV ventricles
Reference values for the transverse dimensions of the third and fourth ventricles (Table 1)[9]
| Gender and value (mm) | Up to 20 years | 21-39 years old | 30-39 years old | 40-49 | 50-59 | Over 60 years |
| III ventricle (V) | 2.73-4.29 | 2.99-4.71 | 3.15-4.69 | 3.15-4,73 | 3.26-4,72 | 2.66-7,42 |
| IV ventricle (V) | 5.97-8.51 | 5.9-8.62 | 6.57-8,93 | 6.66-9.22 | 6.55-9.43 | 5.88-10.14 |
| III ventricle (M) | 2.62-4.36 | 2.48-4.52 | 3.1-4.82 | 4.0-5.96 | 2.83-5.17 | 3.93-8.29 |
| IV ventricle (M) | 5.87-9.17 | 6.18-9.02 | 6.37-9.39 | 6.17-9.85 | 6.21-9.83 | 6.6-9.84 |
Table 1
Bicaudal index
The bicaudal index (BCI) is a commonly used linear measure of the lateral ventricles (Fig. 3). To account for natural changes in ventricular size with aging, (BCI) is then divided by the upper limits of “normal” age to calculate the relative bicaudal index [6–7].
BKI = A/B, where A = width of the frontal horns at the level of the caudate nuclei; B = brain diameter at one level [6-7].
| Age | up to 46 years old | 46-55 years old | 56-65 years old | Over 66 years |
| Meaning | 0.11 | 0.13 | 0.15 | 0.15 |
| Range | 0.10-0.12 | 0.11-0.14 | 0.13-0.16 | 0.14-0.17 |
Table 2 Normal BCI values stratified by age group.
The diagnosis of hydrocephalus is established when the BCI is greater than 1. Normative values determined in subjects without neurological disease in the mid-to-late 1970s [6-7]. Divide the width of the frontal horns of the lateral ventricles, at the level of the caudate nuclei, by the corresponding diameter of the brain. Take the measurement on a section that includes the foramen Monro [6-7].
Fig. 3 Measurement of the bicaudal index (left) and asymmetry of the lateral ventricles in the absence of an organic cause (right).
Causes of enlarged ventricles of the brain
It is believed that changes in ventricular structures in infants are genetically determined. Pathological changes in the brain develop due to chromosomal abnormalities that occur in pregnant women. There are other factors that provoke asymmetry of the ventricles and excessive enlargement of parts of the brain:
- Diseases of infectious etiology that a woman suffered during pregnancy.
- Sepsis, intrauterine infections.
- Entry of a foreign body into the brain structures.
- Pathological course of pregnancy caused by chronic diseases of the mother.
- Premature birth.
- Intrauterine fetal hypoxia: insufficient blood supply to the placenta, increased placental blood flow, umbilical cord varicose veins.
- Long waterless period.
- Rapid birth.
- Birth trauma: strangulation by the umbilical cord, deformation of the skull bones.
Experts also note that the ventricles of the brain in newborns may become enlarged due to the occurrence of hydrocephalus of unknown etiology. Congenital causes that provoke expansion of the ventricles of the head include the growth of neoplasms: cysts, benign and malignant tumors, hematomas.
A traumatic brain injury received by a child during childbirth, cerebral hemorrhage, ischemic or hemorrhagic stroke can also cause enlargement of the ventricles of the baby’s brain.
Asymmetry of the lateral ventricles
Most reports and articles agree that asymmetry of the lateral ventricles in the absence of organic pathology is a normal anatomical variant in most cases (Fig. 3 right).
The prevalence of asymmetry of the lateral ventricles in the study population (patients with complaints of headaches or undergoing a medical examination, without complaints, as well as all of them without identified organic pathology of the brain) was 6.1%. In patients, a larger ventricle was more often found on the left side than on the right side (left = 70.0%, right = 30.0%). The density of various brain regions was similar on both sides in the ALV and control group [3].
According to other data, the prevalence of asymmetry in the size of the lateral ventricle in individuals without signs of an underlying etiology is 5-12% [3-5].
Study conclusion: The clinician should be alert to detect ALV on an unsatisfactory CT scan, especially in cases with severe asymmetry or diffuse ventricular enlargement, and to look for possible associated disorders [3].
In a Canadian study conducted in 1990 with a group of 249 patients, both headaches and seizures were more common in patients with asymmetrical lateral ventricles [4]. In a more recent Turkish study of 170 patients, headache was more common in patients with asymmetrical lateral ventricles than in those with “normal” ones (control group), otherwise there was no significant difference in presentation between the two groups [3].
Asymmetry of the lateral ventricles clearly has an objective cause in patients with the presence of organic pathology, such as: the volumetric effect of an intracranial mass (tumor, inflammatory process or hematoma) and as a result of residual changes leading to stretching of the cavity of the lateral ventricle against the background of the loss of a certain volume of nervous tissue ( so-called ex-vacuo hydrocephalus).
Normal cerebral ventricles in a newborn
Normally, a person has four ventricles in the head: two lateral, they are located symmetrically, the third and fourth, located in the middle. The third is conventionally anterior, the fourth is posterior. The fourth ventricle passes through the cisterna magna, connecting to the central canal (spinal cord).
Why are doctors concerned about enlarged ventricles of the brain? The main function of the lateral structures is the production of cerebrospinal fluid and regulation of the volume of cerebrospinal fluid. A large release of fluid and a violation of its excretion provokes disruption of brain function.
The depth of the third ventricle should normally not exceed 5 mm, the fourth ventricle – 4 mm. If the lateral ventricles of the brain are considered, the norm for a newborn is calculated as follows:
- Anterior horns – from 2 mm to 4 mm.
- Occipital horns – from 10 mm to 15 mm.
- Lateral bodies - no deeper than 4 mm.
The standard depth for a large tank is 3-6 mm. All brain structures should grow gradually, the size of the ventricles should be linearly consistent with the size of the skull.
Volume of the ventricular system
Normal values of the volume of the ventricular system (in Table 3) [8].
| Ventricular system (cm3) | Meaning | RMS deviation |
| Intracranial volume | 1444.6 | 108.1 |
| Right lateral ventricle | 5608.3 | 1310.8 |
| Left lateral ventricle | 6047.2 | 1214.5 |
| Both lateral ventricles | 11655.4 | 2368.1 |
| Third ventricle | 910.5 | 151.3 |
| Fourth ventricle | 1793.6 | 356.9 |
Table 3
Source
- Methodological manual for students of medical institutes. Compiled by: Gubsky L.; Borkina P.; Abovich Y.; Evstifeeva N.; Timakov V. link
- Orlov Yu.A. Hydrocephalus. – Kyiv, 1995. – 75 p.
- Cerebral lateral ventricular asymmetry on CT: how much asymmetry is representing pathology? Kiroğlu Y1, Karabulut N, Oncel C, Yagci B, Sabir N, Ozdemir B.Surg Radiol Anat. 2008 May;30(3):249-55. doi:10.1007/s00276-008-0314-9. Epub 2008 Feb 6. link
- Grosman H, Stein M, Perrin RC, Gray R, St Louis EL. Computed tomography and lateral ventricular asymmetry: clinical and brain structural correlates. (1990) Canadian Association of Radiologists journal = Journal l'Association canadienne des radiologists. 41 (6): 342-6. Pubmed
- Shapiro R, Galloway SJ, Shapiro MD. Minimal asymmetry of the brain: a normal variant. (1986) A.J.R. American journal of roentgenology. 147(4):753-6. doi:10.2214/ajr.147.4.753 - Pubmed
- Gijn, Jan van et al. “Acute Hydrocephalus After Aneurysmal Subarachnoid Hemorrhage.” Journal of Neurosurgery 63.3 (1985): 355-362. link
- Dupont, Stefan and Alejandro A Rabinstein. “CT Evaluation Of Lateral Ventricular Dilation After Subarachnoid Hemorrhage: Baseline Bicaudate Index Balues.” Neurological Research 35.2 (2013): 103-106. link
- Morphometric Changes in Lateral Ventricles of Patients with Recent-Onset Type 2 Diabetes Mellitus. Junghyun H. Lee, Sujung Yoon, Perry F. Renshaw, Tae-Suk Kim, Jiyoung J. Jung, Yera Choi, Binna N. Kim, Alan M. Jacobson, In Kyoon Lyoo. Published: April 4, 2013 “PLOS one”.link
- "Third and Fourth Cerebral Ventricular Sizes among Normal Adults in Zaria-Nigeria." Ahmed Umdagas Hamidu, Solomon Ekott David, Sefiya Adebanke Olarinoye-Akorede, Barnabas Danborno, Abdullahi Jimoh, Olaniyan Fatai. Year:2015, Volume:2, Issue:2, Page:89-92 link