The problems of treating a child with neurological pathology are extremely relevant in our time. This is due to a general decline in the birth rate, an increase in the number of unfavorable factors that provoke damage to the nervous system, and an increased incidence of the birth of unhealthy, physiologically immature children.
Very often, the direct causes of brain damage are hypoxic-ischemic processes as a result of insufficient oxygen supply to the nervous tissue. In ICD-10, the diagnosis is encrypted in several sections. The closest in pathophysiology are codes P21.9 (neonatal anoxia) and G 93.1 (anoxic brain damage, not classified elsewhere).
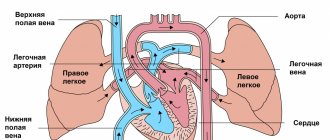
Anoxic damage to the nervous system in children is caused by a lack of adequate oxygen supply to neurons . Under such conditions, the cell quickly changes its functional properties and is not able to function fully. Subsequently, the morphology of neurons is also disrupted. Oxygen deficiency leads to cellular necrosis and/or apoptosis and forms foci of ischemia in the brain. Symptoms of cerebral anoxia can be severe and fatal.
Neurons begin to die after only 4 minutes of acute anoxia. Under conditions of hypothermia, this time is extended to 20-30 minutes, and at high temperatures it is reduced to 120 seconds.
Description of the disease
The problems of treating children with neurological pathologies are extremely relevant in our time. This is directly related to the general decline in the birth rate, and in addition, to the increase in the number of various unfavorable factors that provoke damage to the children's nervous system. Among other things, this is largely due to the fact that in the modern world, cases of the birth of unhealthy, and at the same time physiologically immature, children have become more frequent.
Very often, the main causes of anoxic brain damage are hypoxic and ischemic processes due to insufficient oxygen supply to nerve tissues. In the ICD-10 system, such a diagnosis is encrypted in several sections at once. The closest in pathophysiology are codes P21.9 (it implies neonatal anoxia) and G93.1 (in this case we are talking about anoxic brain damage, not classified in other categories).
Etiology and pathogenesis
There are a number of unfavorable factors that can lead to the development of anoxic damage to the nervous system. Even minimal deviations significantly disrupt the functioning of the brain due to the fact that they affect immature nervous tissue. Subsequently, this may manifest itself as a neurological deficit, a slowdown in the rate of formation of cerebral zones and centers, and a delay in overall development. Prolonged anoxia leads to death or the formation of a vegetative state .
The root causes of anoxia may include acute thrombosis, suffocation, strangulation, drowning, electric shock, cardiac arrest, alcohol or drug intoxication, neuroinfections, as well as other factors that prevent the supply of oxygen to the brain. Separately, anoxic lesions of the nervous system of the perinatal period are distinguished. This is facilitated by:
- pathological course of pregnancy (somatic diseases of the mother, gestosis, threat of miscarriage, symptoms of qualitative and quantitative starvation, intoxication, general immaturity of the pregnant woman, etc.);
- intranatal (arising during childbirth) damaging factors. This includes symptoms of premature abruption, placenta previa, entanglement of the umbilical cord around the fetal neck, umbilical cord nodes, premature and late, rapid and protracted labor, weakness of labor;
- postnatal (postpartum) disorders. These include meconium aspiration, repeated apneas, cardiovascular defects, sepsis, and hemolytic disease of the newborn.
All of the above provocateurs cause the development of foci of ischemia. In parallel, as a compensatory reaction, the permeability of cerebral vessels increases. On the one hand, this reduces cerebral perfusion and aggravates ischemia, on the other, it serves as one of the mechanisms for the development of hypoxic-hemorrhagic lesions. Due to it, the process of diapedetic impregnation of red blood cells begins through the altered vascular wall. In addition, under conditions of oxygen starvation, glucose utilization occurs along the anaerobic pathway with the formation of lactate. During perinatal anoxia, acid compounds irritate the digestive and respiratory centers of the brain stem. During childbirth, this provokes premature passage of meconium and its aspiration into the child’s respiratory tract, which contributes to even greater hypoxia.
Morphologically, deviations are observed in the form of:
- cerebral edema (focal or multifocal);
- ischemic lesions of brain tissue, basal ganglia, thalamus, cerebellum;
- cortical and subcortical small focal necrosis;
- periventricular leukomalacia.
Classification
Depending on the predominant morphological result of the development of disorders, anoxic pathology can manifest itself in the form of cerebral ischemia, intracranial hemorrhages of hypoxic origin, and combined non-traumatic ischemic-hemorrhagic lesions of the central nervous system.
The mechanism of development of anoxia allows us to classify it into the following types:
- anoxic, formed as a result of the cessation of oxygen supply through the respiratory tract;
- anemic, occurring as a result of massive blood loss, vascular spasm, thrombosis;
- congestive, which is a consequence of cerebral circulation discirculation;
- metabolic – a manifestation of metabolic disorders.
In addition, there is acute anoxia, which develops suddenly, and a chronic form of pathology with a gradual increase in oxygen deficiency (hypoxia).
The duration of the decrease in oxygen supply determines the gradation of anoxia into mild (oxygen starvation for up to 80 seconds), moderate (up to 120 seconds) and severe (up to 240 seconds) forms. Such a division is quite arbitrary, since the severity of anoxic manifestations will depend on the ambient temperature, the age of the patient and the condition of the body itself.
Clinic
Clinical symptoms are primarily determined by the cause of anoxia and the duration of its effects. Acute anoxia is manifested by loss of consciousness, which may be accompanied by convulsive paroxysms. Subsequently, profound amnesia occurs. Severe and moderate forms of anoxia provoke persistent neurological disorders:
- Paralysis and paresis;
- Sensitivity disorders;
- Cognitive impairment;
- Vestibulocerebellar syndrome;
- Epileptic seizures.
Severe anoxic lesions can lead to decortication syndrome - functional shutdown of the cerebral cortex, and the development of a vegetative state.
Anatomical picture of this disease
Unfortunately, medicine has not yet identified the exact mechanism of anoxic brain damage. True, the anatomical picture of this pathology is quite simple. The fact is that the nervous tissue ceases to receive oxygen in sufficient quantities; against the background of this, a hypoxic ischemic process occurs, which, even over a short period of time, is detrimental to the structure of the brain.
In other words, each neuron, as it were, does not receive the required amount of oxygen during the blood supply. Neurons in children are not yet as developed as they are in adults, so their relationship with the brain is only at the stage of formation. With little supply to the cell, it simply ceases to function correctly, and at the same time changes morphologically and internally.
Accordingly, anoxia is called a morphological degenerative process that has an extremely detrimental effect on healthy tissue. Symptoms of anoxic brain damage in children are extremely difficult to tolerate, which often ends in death. Next, we will figure out what causes are the provoking factors for the occurrence of this disease in children and newborns.
Diagnostics
Acute anoxia can be diagnosed based on the results of a survey of the patient himself, his relatives or nearby people. The doctor finds out the cause of this condition and, if possible, the duration of exposure to the traumatic factor. Additional diagnostic methods are:
- computed and magnetic resonance imaging;
- electroencephalogram;
- single photon emission computed tomography;
- assessment of evoked auditory and visual potentials.
Causes of brain pathology
A single root cause for the occurrence of anoxic brain damage has not yet been identified. But it is worth noting that there are a lot of provoking factors that can precede such a terrible phenomenon. These factors can interfere with normal blood supply, and in addition, the supply of the required amount of oxygen to the child’s brain:
- We are talking about cardiac arrest or suffocation.
- The impact of intoxication with chemicals, for example, is sometimes even influenced by dirty ecology. It is worth noting that the child’s body is very sensitive to the cleanliness of the environment.
- Various viruses along with neuroinfections.
- Receiving sunstroke (or heatstroke) and electric shock.
- Performing surgery on the heart or brain.
- The onset of coma or clinical death.
- The effect of prolonged arterial hypotension (i.e. decreased blood pressure).
Symptoms of this dangerous pathology
Anoxic brain damage in newborns is usually extremely severe. The fact is that even the onset of short-term hypoxia can cause an attack of suffocation along with convulsions and internal necrosis. General symptoms and signs of anoxia include:
- The child has epileptic seizures and convulsions.
- The presence of involuntary trembling of the limbs.
- The occurrence of sensory disturbances.
- The appearance of malfunctions in the functioning of the organs of hearing and vision.
- The occurrence of photophobia and increased photosensitivity.
- The appearance of paralysis and paresis of the limbs.
- The occurrence of asthma attacks and breathing problems.
- The appearance of a heart rhythm disturbance.
- The occurrence of headaches.
Diagnosis of this brain pathology
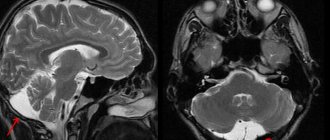
Diagnosis of anoxic brain damage in children involves, first of all, magnetic resonance and computed tomography. Electroencephalography may also be needed. Based on the results of all these procedures, the doctor can establish the correct diagnosis and predict the further course of the disease.
Treatment usually includes two successive stages. Firstly, it is the removal of the root causes of the disease along with the restoration of the body. At this stage, it is necessary to understand what exactly caused anoxia in order to eliminate it. And directly at the second stage, taking vitamins is required along with breathing exercises and taking vascular medications in order to restore the functioning of the heart and blood vessels, among other things.
How and where is anoxic brain damage treated?
Premature birth is a leading cause of infant mortality and a significant factor in the loss of human potential of surviving children during later life. According to foreign authors, several million children with very low body weight (VLBW) are born every year around the world [1]. In the United States, 90% of the 65,000 newborn VLBW infants survive the neonatal period due to great advances in intensive care, but 5–10% of these children are later diagnosed with cerebral palsy [2].
The introduction of modern medical technologies in the last decade has been marked by a decrease in perinatal and infant mortality. At the same time, an increase in the survival rate of children with VLBW and extremely low body weight (ELBW) at birth entails an increase in morbidity and the formation of early disability [3]. Among the causes of childhood disability, pathology of the nervous system ranks first, and the contribution of perinatal lesions reaches 60–80% of all neurological diseases [4]. In Russia, annually no more than 2.5-5% of those examined as disabled since childhood are recognized as able to work, compared to 50% abroad [5].
Among the factors that adversely affect the antenatal period, disruption of the uteroplacental circulation, which can be caused by both extragenital and somatic pathology of the mother, is of great importance [6]. Disturbances in the uteroplacental blood flow, in turn, lead to the development of hypoxia, which is the central link in the pathogenesis of antenatal damage to the fetus, and primarily to the central nervous system (CNS). The works of many authors have established a whole complex of physiological adaptive reactions of the fetus to unfavorable developmental conditions, in particular to hypoxia [7-10]. However, there is insufficient information on the biochemical status of the nervous tissue of the fetus and newborn in this pathology. Of great interest in this regard is the study of glucose metabolism, the characteristics of free radical oxidation and glutamate metabolism, the processes of necrosis and apoptosis.
In utero, the fetus is in a state of hypoxia, but this environment is physiological for it, moreover, in the early stages of the embryonic period it is necessary for normal cell differentiation. Fetal oxygenation depends on oxygen partial pressure gradients between maternal and placental blood, fetal blood and fetal tissue. It is known that in the first weeks after conception in the embryonic period, the level of partial pressure of oxygen (pO2) is extremely low and amounts to about 18-20 mm Hg. Presumably, this is necessary to protect the embryo, which is very sensitive to the damaging effects of reactive oxygen species [11]. Hypoxia in the embryonic period causes angiogenesis and is a prerequisite for maintaining pluripotency of stem cells [12]. It is noteworthy that in the first trimester of pregnancy, embryonic stem cells develop at a pO2 level of about 10-15 mm Hg, while in the endometrium, pO2 is about 25 mm Hg. Stem cells demonstrate more efficient growth and differentiation at low oxygen pressures of 10-15 mmHg. [13]. Prolonged hypoxia will stimulate angiogenesis through transcriptional and post-transcriptional regulation of growth factors: vascular endothelial growth factor, erythropoietin, placental growth factor and angiopoietin1 [14].
The main regulator of adaptive cell responses to hypoxia is hypoxia-inducible factor 1 (HIF-1), a heterodimeric transcription factor including subunits (HIF-1α and IGF-1β). IGF-1α stabilizes when the oxygen concentration is below a certain critical threshold, thus accumulating in a hypoxic environment. IGF-1β is present in the cell nucleus, and under hypoxic conditions it dimerizes with IGF-1α, improving oxygen delivery to the tissue [15, 16]. At the 14-16th week of pregnancy, pO2 rises to stable values of 45-50 mmHg. and remains so until the end of pregnancy. In late pregnancy, the rate of cell proliferation and differentiation decreases [17, 18]. Lipid peroxidation processes are present from the very beginning of pregnancy, which contributes to the normal development of the fetus. At the end of the first trimester, physiological oxidative stress causes regression of the villi that were formed throughout the surface of the chorionic sac to form the final discoid placenta [19]. The postnatal increase in oxygen concentration causes a surge in the formation of its reactive species, with the expression of antioxidant enzymes such as superoxide dismutase, catalase and glutathione peroxidase dynamically increasing during the last weeks of pregnancy. Similarly, the availability of the most important non-enzymatic antioxidants increases: glutathione, heme oxygenase, vitamins C and E, β-carotenes, etc. [20]. The premature infant is at greater risk of free radical damage [21]. The use of high concentrations of oxygen during neonatal resuscitation is thought to cause hyperoxemia. At the same time, a significant correlation was found between oxidized glutathione (GSSG), pO2 and the activity of enzymes in the glutathione redox cycle [22, 23]. Many diseases associated with prematurity, such as retinopathy, bronchopulmonary dysplasia, and intraventricular hemorrhage, are associated with free radical damage as a result of the immaturity of the antioxidant system of preterm infants [24].
Nervous tissue is most vulnerable when exposed to hypoxia. Hypoxia leads to disruption of the exchange of oxygen and carbon dioxide, which in turn causes metabolic disorders and hemodynamic disorders [25]. The following mechanisms underlying cerebral damage during hypoxia-ischemia are known: local disturbances in the metabolism of high-energy compounds, excessive lipid peroxidation and disturbance of Na+/K+-ATPase activity, extracellular accumulation of K+ and intracellular accumulation of Ca2+, intracellular acidosis, disturbance of neurotransmitter metabolism [26 ]. The main links of hypoxic-ischemic stress are presented by P. Marro [27].
- Lack of oxygen. A deficiency of oxygen as an electron acceptor in tissues leads to disruption of electron transport in the Krebs cycle and respiratory chain, replenishment of energy by increasing cerebral blood flow and anaerobic metabolism [28].
— Glutamate-calcium cascade. An increase in glutamate concentration activates N-methyl-D-aspartate (NMDA) receptors, which is accompanied by an increase in intracellular Ca2+ [29]. Disturbances in mitochondria and endoplasmic reticulum can lead to further accumulation of intracellular Ca2+. An increase in Ca2+ concentration inside the cell promotes the formation of free radicals, which in turn causes lipid peroxidation of the cellular and intracellular membranes. Along with this, the accumulation of intracellular Ca2+ is naturally accompanied by an increase in its concentration in the cell nucleus. Excess intranuclear Ca2+ is a factor in the activation of protoapoptotic genes, which trigger genetically programmed cell death - apoptosis [30].
— The role of free radicals. Hypoxia-ischemia causes inadequate saturation of mitochondrial cytochrome oxidase, disruption of electron transport in mitochondria, which leads to an increase in the concentration of superoxide anion and the entry of free radicals from mitochondria into the cytoplasm [31]. An increase in intracellular Ca2+ concentration activates NO synthetase, cyclooxygenase and lipoxygenase, which promotes the formation of free radicals. Their excess leads to additional release of excitatory amino acids and activation of NMDA receptors [32].
- Inflammatory factors. The effect of hypoxia-ischemia on microglia promotes the synthesis of cytokines, interleukin-1β (IL-1β), tumor necrosis factor α (TNFα) [33]. IL-1β activity is accompanied by the production of specific proteases and the development of apoptosis. Excessive production of TNFα has a direct toxic effect and causes vascular infiltration with the release of cytotoxic factors, reactive oxygen species and cytokines [34].
— The role of nitric oxide (NO). NO synthetase is found in endothelial cells, astrocytes, and neurons. There are 3 isoforms of NO synthetase: neuronal (regulates synaptogenesis and remodeling and depends on Ca2+); endothelial (regulates vascular tone, especially vasodilation, and depends on Ca2+); inducible (present in macrophages and astrocytes, induced by cytokines, independent of Ca2+) [35]. Activation of NMDA receptors causes the production of neuronal NO synthetase, which promotes the formation of nitric oxide (NO.) radical and damage to neuronal DNA [36].
- Apoptosis. The processes described above develop in the first minutes of acute hypoxia, after which the apoptosis mechanism is activated [37]. Hypoxia, through a number of pathogenesis links, promotes the accumulation of intracellular Ca2+, activation of endonucleases, and damage to gene expression. This leads to disinhibition of the phagocytic activity of glial cells and neurons, which phagocytose the damaged neuron, causing a decrease in its size and sequestration [38].
The most significant loss of nervous tissue cells develops 2-6-48 hours after birth, due to pathological oxidative stress. Under such conditions, in the first hours and days of life after birth, newborns who have suffered hypoxia develop a pronounced imbalance in the cerebral circulation regulation system, which aggravates the course of the ischemic process [39].
A feature of premature infants is the immaturity of the antioxidant system, since a physiological increase in antioxidant capacity occurs at the end of pregnancy, which is why they are more susceptible to oxidative stress, especially when their condition requires respiratory therapy [40]. In this regard, there is a need to study oxidative stress in these children, in particular by measuring lipid peroxidation products and components of the antioxidant system.
Antioxidants are known to have anti-inflammatory activity, and the glutathione system is considered a critical factor in the development of inflammation and immune responses [41, 42]. This is confirmed by changes in the levels of cytokines, acute phase proteins and glutathione during inflammation [43, 44]. The glutathione system includes its forms, a number of enzymes for its synthesis and catabolism, and transport mechanisms. All these components make an important contribution to changes in glutathione status [45].
When studying the classification of cerebral lesions in newborns, it should be noted that the most popular among neonatologists is the classification of hypoxic encephalopathy according to H. Sarnat and M. Sarnat [46]. It combines clinical signs of cerebral ischemia and electroencephalography (EEG) results. This classification evaluates the main indicators of a newborn: level of consciousness, neuromuscular status, reflexes, autonomic function, presence of seizures, EEG. Depending on the severity of cerebral dysfunction, stage I, II or III of encephalopathy is established. Canadian neonatologists modified the Sarnat classification, adding thermoregulation disorders and excluding EEG and some other indicators [47]. Neonatologists in Great Britain use the classification of hypoxic cerebral disorders by L. Dubowitz et al.[48]. In the International Classification of Diseases, 11th revision (ICD-11), hypoxic-ischemic brain lesions in newborns belong to the group of “neurological disorders characteristic of the perinatal and neonatal periods” [49]. The main difference from the classification of the 10th revision is that in ICD-11 this group is supplemented with diseases that were not previously identified separately. For example, perinatal arterial stroke and neonatal cerebral sinovenous thrombosis. At the same time, congenital hydrocephalus was excluded from the proposed classification. The diagnosis of newborn asphyxia was placed in the “group of other disorders arising in the perinatal period.” At the same time, newborn asphyxia with an Apgar score of 0-3 points and newborn asphyxia with an Apgar score of 4-6 points were separately identified. As for cerebral injuries of a hypoxic-hemorrhagic nature, they were classified into the group of “hemorrhagic and hematological disorders in the fetus and newborn.” At the same time, the classification of intraventricular hemorrhages has changed somewhat. In ICD-11, it is customary to distinguish 4 degrees of intraventricular hemorrhage, while in ICD-10 the 3rd and 4th degrees were combined.
Symptoms of severe central nervous system damage may not appear immediately after birth, but may occur several hours later. However, clinical symptoms do not always reflect the true severity of the disease. In this regard, intravital assessment of changes that occur in the cells of nervous tissue in the early neonatal period is of particular relevance. Ultrasound examination (ultrasound) allows us to identify structural changes in the brain of newborns due to perinatal hypoxic damage to the central nervous system. Analysis of ultrasound and pathomorphological data indicates that the nature of ischemic brain damage depends not only on the severity of perinatal hypoxia, but also on the maturity of the child [50]. In full-term newborns, cerebral ischemia is accompanied by the occurrence of selective neuronal necrosis, subcortical and multicystic encephalomalacia, and cerebral infarctions [51]. Severe perinatal hypoxia in premature infants 34–37 weeks of gestation, as a rule, leads to the development of periventricular leukomalacia. In connection with the nursing of extremely premature infants with ELBW, ultrasound has become more likely to detect such forms of ischemic damage as diffuse leukomalacia and periventricular hemorrhagic infarction. It should be noted that in the first 24-48 hours of the debut of neonatal arterial ischemic stroke, this method does not have sufficient sensitivity and specificity, since the focus of ischemic damage begins to appear only on the 2-3rd day from the onset of ischemia, which is associated with the course of pathohistological processes. The severity of the inflammatory reaction, the intensity of necrosis and apoptosis reach their peak 48–72 hours after circulatory disturbance [52]. This leads to a change in the echogenicity of the damaged brain parenchyma. Further assessment of the evolution of the ischemic focus is not inferior in information content to magnetic resonance imaging (MRI) [53, 54]. The use of Doppler ultrasound helps to increase the sensitivity of ultrasound in the early stages of cerebral damage. Duplex scanning allows you to objectively assess the hemodynamic characteristics of cerebral vessels. The value of Doppler ultrasound technique in the acute period of the disease is the identification of the vasodilation phase, the earliest sign of ischemic damage, which occurs within 30 minutes after the development of a vascular accident and persists for the first 5-6 days [55]. Vasodilation develops in response to the action of various metabolites and increases the supply of glucose and oxygen to ischemic tissue. Its characteristic features are an increase in blood flow velocity and a decrease in peripheral resistance indices in the damaged vascular system [56, 57]. MRI has become the most informative method for diagnosing perinatal brain damage. The presence of various pulse sequences provides high sensitivity and specificity, even in the early stages of the development of a vascular accident [58, 59]. MRI with diffusion-weighted images and the construction of maps of the measured diffusion coefficient (MCD) makes it possible to detect an ischemic lesion within 30 minutes from the moment of its occurrence. ICD serves as a quantitative characteristic of diffusion in tissue and reflects the presence of intracellular edema [60]. The presented diagnostic methods are necessary to identify, determine the localization, severity of brain damage and prognosis. Their disadvantages are the short diagnostic time interval and the limited possibility of repeated examination [61]. Over the past 20 years, the diagnostic value of biomarkers has been studied to predict the outcome of cerebral injury in newborns [62, 63]. Considering the pathophysiological changes that occur as a result of damage to brain tissue, neuroproteins, calcium-binding protein, vasoactive substances, markers of oxidative stress, inflammatory mediators, etc. have been studied in detail [64-67]. However, despite the promise of studying biomarkers, there is no data on their practical use in medicine.
Treatment of hypoxic brain lesions is the subject of heated debate and extreme opinions, ranging from complete denial of the need for treatment with neurotropic drugs to aggressive polypharmacy [26]. According to modern views, hypoxic-ischemic encephalopathy occurs during asphyxia, usually in the structure of multiple organ disorders, therefore the main principle of therapy is to remove the child from asphyxia and maintain vital functions [68]. Specific treatment for hypoxic-ischemic encephalopathy is therapy for cerebral edema and neuroprotection, which primarily involves controlling the volume of cerebrospinal fluid (CSF), cerebral perfusion and the volume of brain matter. Cerebral perfusion depends on arterial inflow, venous outflow and the metabolic rate of nervous tissue. At this stage, the adequacy of artificial ventilation and the effectiveness of hypothermia are of great importance. It should be noted that hypothermia is carried out in newborns with a gestational age of at least 36 weeks if at least one of the signs set out in the special criteria is present [69]. Control of fluid volume in the CSF pathways is achieved by inhibiting the production of CSF and improving its outflow. Controlling brain volume involves enhancing active transport and stabilizing neuronal membranes. It should be noted that at the moment the use of drugs in neonatology is limited due to the high risk of side effects and lack of evidence base. However, the search for such drugs continues to this day. In particular, over the past two decades there have been significant changes in the understanding of the role of erythropoietin as a neuroprotector. Erythropoietin has been shown to be involved in neurogenesis and angiogenesis during embryonic development and is activated after brain injury [70], and also has a cytoprotective effect on endothelial, glial cells and neurons [71]. The neuroprotective role of erythropoietin was first identified in several in vitro
and
in vivo
[72]. At the same time, it was found that erythropoietin has anti-apoptotic [73], antioxidant [74] and anti-inflammatory effects [75]. In addition, it attenuates the effects of inflammation by reducing reactive astrocytosis and suppressing microglial activation, and reduces the number of immune cells at the site of inflammation [76]. The absence of endogenous erythropoietin is known to increase ischemic brain damage and worsen neuronal survival [77]. The established protective effects of erythropoietin during ischemia and reperfusion have prompted the use of recombinant erythropoietin in premature infants with cerebral ischemia, intraventricular hemorrhage, and periventricular leukomalacia [78]. Thus, one study assessed the effectiveness and safety of erythropoietin in neonatal hypoxic-ischemic encephalopathy. This study included 167 newborns with moderate to severe cerebral damage. All children were divided into two groups. Children of the first group received standard therapy for hypoxic-ischemic encephalopathy, and children of the second group received erythropoietin at a dosage of 300 and 500 U/kg to standard therapy. The drug was administered in the first 48 hours every other day for 2 weeks. It turned out that mortality and disability were 19.2% more common in the group of children who did not receive erythropoietin [79].
Thus, the problem of ischemic cerebral damage in premature newborns is very relevant. First of all, this is confirmed by the high incidence of this pathology and the high risk of death and disability in children. The question of complex diagnosis of cerebral disorders in premature infants remains open, because there is no single algorithm that combines data from the clinical picture, instrumental and laboratory research methods. Of great interest is the search for predictors of unfavorable outcome in order to optimize treatment approaches.
The authors declare
no conflict of interest.
The authors declare no conflicts of interest.
Information about authors
Anuriev A.M. - https://orcid.org/0000-0002-6724-5067; e-mail
Gorbachev V.I. — https :// orcid . org /0000-0001-6278-9332
How to quote:
Anuriev A.M., Gorbachev V.I. Hypoxic-ischemic brain lesions in premature newborns. Journal of Neurology and Psychiatry. S.S. Korsakov.
2019;119(8 issue 2):63-69. https://doi.org/10.17116/jnevro201911908263
Corresponding author:
— Anuriev Alexey Mikhailovich — e-mail
Methods to combat this disease
So, as has already become clear, the treatment of the disease that has arisen usually involves several stages. In case of an acute illness, it is urgently necessary to completely eliminate the influence of factors leading to anoxia:
- The child requires sanitation of the respiratory tract.
- Removal of a foreign body.
- It is necessary to remove the patient from the area of exposure to carbon dioxide.
- Strangulation must be stopped.
- Preventing the effects of electric current.
At this stage, it is necessary to maintain normal blood circulation and oxygen supply; in some cases, artificial respiration devices are used. In this case, support is provided at a level that should not allow irreversible changes in the brain. If natural breathing is preserved, the child requires oxygen inhalation and transportation to the hospital. If breathing is ineffective, intubation will be required.
Hypoxic lesions of the central nervous system
Section I
Hypoxic lesions of the central nervous system
I. A) P 91.0 Cerebral ischemia (hypoxic-ischemic encephalopathy, perinatal hypoxic brain damage)
Cerebral ischemia grade I (mild)
a) Intrapartum hypoxia, mild asphyxia at birth;
b) Excitation of the central nervous system is more common in full-term infants, depression - in premature infants, lasting no more than 5-7 days;
c) Moderate hypoxemia, hypercarbia, acidosis;
NSG, CT, MRI - without pathological abnormalities;
DEG - compensatory increase in speed along the main arteries of the brain;
Diagnosis example:
P 91.0 “Cerebral ischemia stage I” or “Hypoxic-ischemic damage to the central nervous system stage I”
Cerebral ischemia degree II (moderate)
a) Factors indicating intrauterine fetal hypoxia; moderate asphyxia at birth; extracerebral causes of cerebral hypoxia that arise postnatally;
b) CNS depression, excitation or change of phases of cerebral activity (lasting more than 7 days);
Convulsions in premature infants are often tonic or atypical (convulsive apnea, stereotypical spontaneous oral automatisms, fluttering of the eyelids, myoclonus of the eyeballs, “rowing” movements of the arms, “pedaling” of the legs); in full-term infants - multifocal clonic; Attacks are usually short-term, single, less often repeated;
Intracranial hypertension (transient, more often in full-term infants);
Autonomic-visceral disorders;
c) Metabolic disorders (hypoxemia, hypercarbia, acidosis are more pronounced and persistent)
NSG - local hyperechoic foci in the brain tissue (in premature infants, more often in the periventricular region; in full-term infants, subcortically.
MRI - focal lesions in the brain parenchyma are determined in the form of changes in the nature of the magnetic resonance signal on T1 and T2-weighted images.
CT scan of the brain shows local foci of low density in the brain tissue (in premature infants, more often in the periventricular region; in full-term infants, subcortically and/or cortically.
DEG are signs of hypoperfusion in the middle cerebral artery in full-term infants and the anterior cerebral artery in premature infants. An increase in the diastolic component of blood flow velocity, a decrease in the resistance index.
Diagnosis example:
P 91.0 “Cerebral ischemia degree II” or “Hypoxic-ischemic damage to the central nervous system II degree.”
In cases of diagnosis of specific structural changes in the brain, an additional code is set (for example, P 91.2 cerebral leukomalacia of a newborn).
Cerebral ischemia grade III (severe)
a) Factors leading to intrauterine fetal hypoxia and/or severe perinatal asphyxia; extracerebral causes of persistent cerebral hypoxia (CHD, severe forms of SDR, hypovolemic shock, etc.);
b) Progressive loss of cerebral activity - over 10 days
(in the first 12 hours of life there is deep depression or coma, in the period from 12-24 there is a short-term increase in the level of wakefulness, from 24-72 hours there is an increase in depression or coma
- Repeated convulsions, epistatus is possible.
- Dysfunction of the brain stem (disturbances in breathing rhythm, pupillary reactions, oculomotor disorders).
- The position of decortication or decerebration (depending on the extent of the lesion).
- Severe autonomic and visceral disorders.
- Progressive intracranial hypertension.
c) Persistent metabolic disorders.
NSG - a diffuse increase in the echogenicity of the brain parenchyma - is characteristic of full-term infants. Increased echogenicity of periventricular structures is typical for premature infants. Narrowing of the lateral ventricles. Subsequently, cystic periventricular cavities (PVC) are formed in premature infants, and signs of atrophy of the cerebral hemispheres with passive expansion of the cerebrospinal fluid spaces appear.
CT scan - decreased density of brain parenchyma, narrowing of the cerebrospinal fluid spaces, multifocal cortical and subcortical foci of reduced density, changes in the density of the basal ganglia and thalamus - mainly in full-term infants, periventricular cystic cavities - in premature infants (Check with a radiologist)
MRI - lesions in the brain parenchyma are detected as changes in the magnetic resonance signal on T1 and T2-weighted images.
DEG - paralysis of the main arteries of the brain, with transition to persistent cerebral hypoperfusion. Decrease in diastolic blood flow velocity, change in the nature of the curve (its leasing or pendulum-like nature). Increase in resistance index.
Diagnosis example:
P 91.0 “Cerebral ischemia of the third degree” or “Hypoxic-ischemic damage to the central nervous system of the third degree.”
In cases of diagnosis of specific structural changes in the brain, an additional code is set (see Appendix).
I. B) R 52 INTRACRANIAL HEMORRHAGES
(hypoxic, non-traumatic)
P 52.0 Intraventricular hemorrhage of the 1st degree (subependymal)
a) Ante- and - intrapartum hypoxia, mild asphyxia at birth, repeated attacks of apnea, jet administration of hyperosmolar solutions.
b) Develops predominantly in premature or immature newborns Course: asymptomatic, absence of specific neurological disorders
c) Transient metabolic disorders
NSG - hyperechoic areas, unilateral or bilateral localization in the thalamo-caudal notch or in the region of the head of the caudate nucleus. The time frame for transformation of a subependymal hematoma into a cyst is 10-14 days or more.
CT, MRI - do not have diagnostic advantages over NSG.
DEG - without pathological changes.
P 52.1 Intraventricular hemorrhage of the second degree
(subependymal + intraventricular)
Develops predominantly in premature infants (35-65%).
a) Factors indicating intrauterine fetal hypoxia and/or moderate asphyxia at birth. Defects in the provision of primary resuscitation care, arterial hypertension or fluctuation of systemic blood pressure due to SDR, iatrogenic factors (inadequate mechanical ventilation regimens, rapid administration of large volumes or hyperosmolar solutions, functioning fetal communications, pneumothorax, etc.). Coagulopathies.
b) There are 2 main variants of the flow: gradual (wavy) and catastrophic.
- Catastrophic course: short-term motor excitation is suddenly replaced by progressive depression of cerebral activity with transition to coma. Deep apnea, increasing cyanosis and marbling of the skin. Tonic convulsions, oculomotor disorders, bradyarrhythmia, and thermoregulation disorders indicate increasing intraventricular hypertension.
- Gradual course (wavy): periodic changes in phases of cerebral activity, attacks of repeated apnea, muscle hypotension, atypical convulsive attacks.
c) Fluctuation and then a rapid decrease in systemic blood pressure (cf. blood pressure < 30 mm Hg)
Fall in hematocrit and decrease in hemoglobin level
Metabolic disorders: hypoxemia, hypercabia, acidosis, hypocalcemia, fluctuations in serum glucose levels
CSF - with an admixture of blood (the time of occurrence of bleeding and its intensity is judged by the microscopic characteristics and the number of red blood cells), reactive pleocytosis, increased protein levels, decreased glucose. During lumbar puncture, blood pressure is often elevated.
NSG changes depend on the time of the study; at the initial stages, hyperechoic zones are determined in the area of the germinal matrix, then ventriculomegaly develops, and subsequently echo-positive formations (thrombi) are visualized in the lumens of the ventricles. In some cases, blockage of the cerebrospinal fluid pathways with the development of acute hydrocephalus is possible.
CT, MRI, PET - do not have diagnostic advantages in the neonatal period over NSG
DEG - fluctuation of blood flow in the main arteries of the brain before the development of intravetricular bleeding, stabilization - after hemorrhage, with the progression of ventriculomegaly (usually after 10-12 days) - increasing hypoperfusion.
P 52.2 Intraventricular hemorrhage of the third degree
(subependymal + intraventricular + periventricular)
Among all variants of IVH, their share accounts for 12 to 17%.
b) Most often observed in premature infants with extremely low body weight.
- Typically catastrophic. Rapid depression of cerebral activity with the development of coma, progressive disorder of vital functions (bradycardia, arrhythmia, apnea, respiratory rhythm pathology).
- Tonic convulsions and oculomotor disorders occur due to dislocation of the brain stem. High incidence of deaths in the first days of life.
c) Severe, difficult to correct, metabolic disorders (hypoxemia, hypercarbia, acidosis, electrolyte disturbances), disseminated intravascular coagulation syndrome.
A progressive fall in systemic blood pressure and cardiac arrhythmias. Critical drop in hematocrit and hemoglobin levels
CSF - with a significant admixture of blood (the time of occurrence of bleeding and its intensity is judged by the microscopic characteristics and the number of red blood cells), reactive pleocytosis, increased protein levels are often observed, and cerebrospinal fluid pressure is often increased.
Diagnostic lumbar puncture is performed according to strict indications and with extreme caution, due to the high risk of herniation of the brain stem into the foramen magnum.
NSG is an extensive hyperechoic area of periventricular localization (hemorrhagic infarction - often unilateral in the fronto-parietal region), the lateral ventricle on the side of the hemorrhage is practically not visualized, later ventriculomegaly and deformation of the lateral ventricle are revealed due to the formation of a porencephalic post-hemorrhagic cystic cavity. Thrombi are often visualized in the lumen of the ventricles, in combination with pronounced dilatation of the ventricular system. Subsequently, an increase in the echogenicity of the ventricular walls is caused by the development of aseptic ventriculitis and hemosiderosis of the periventricular parenchyma. In a significant percentage of cases, occlusive hydrocephalus is formed.
CT, MRI, PET - do not have diagnostic advantages in the neonatal period over NSG
DEG - in the initial stages, a decrease in systolic and diastolic blood flow rates, an increase in the resistance index
Reduced diastolic blood flow velocity, decreased resistance index.
P 52.5 Primary subarachnoid hemorrhage (non-traumatic)
The frequency is about 20%, of which 75% are in premature and immature
a) Intranatal hypoxia, asphyxia at birth. Short gestation period, immaturity. Coagulopathies.
b) Clinical course options:
- Asymptomatic,
- Excitement syndrome with hyperesthesia and acute intracranial hypertension (tension and bulging of the large fontanel, divergence of the sagittal and coronal sutures, profuse regurgitation, intermittent Graefe's symptom);
- Convulsions that suddenly occur on the 2-3rd day of life (focal clonic - more often in full-term infants), atypical convulsions (in premature infants).
c) Metabolic disorders are not typical
NSG is not very informative for diagnosing PSC. In some cases, the expansion of the Sylvian fissure and/or interhemispheric fissure is visualized.
CT and MRI - accumulation of blood is detected in various parts of the subarachnoid space, but more often in the temporal regions.
DEG is not very informative (primary and secondary vasospasm).
CSF - increased pressure, increased erythrocyte content (including changed ones), increased protein concentration, neutrophilic pleocytosis.
R 52.4 Hemorrhage into the substance of the brain (non-traumatic)
parenchymal
P 52.6 hemorrhage in the cerebellum and posterior cranial fossa
(rare)
a) Intrauterine fetal hypoxia, severe or moderate asphyxia at birth. Coagulopathies. Prematurity. Vascular malformations.
b) The clinical picture depends on the location and volume of the hemorrhagic infarction.
- With scattered petechial hemorrhages of subcortical localization, an asymptomatic course is possible.
- With extensive parenchymal hematomas of hemispheric localization, the clinical course is similar to stage III IVH. Progressive loss of cerebral activity with transition to stupor or coma, focal neurological symptoms contralateral to the lesion (distinct asymmetry of muscle tone, focal or tonic convulsions, oculomotor disturbances, etc.); increasing intracranial hypertension.
- Hemorrhages in the posterior cranial fossa and cerebellum are characterized by increasing signs of intracranial hypertension (tension of the fontanelles, dehiscence of the nuchal suture, agitation, frequent tonic convulsions) and brainstem disorders (respiratory, cardiovascular disorders, oculomotor disorders, bulbar syndrome).
c) Severe metabolic disorders that are difficult to correct (hypoxemia, hypercarbia, acidosis, disseminated intravascular coagulation) are usually accompanied by massive hematomas. A progressive increase in systemic blood pressure is subsequently replaced by a fall. Heart rhythm disturbances. Decreases in hematocrit and hemoglobin levels correlate with the amount of bleeding.
CSF - increased pressure, increased erythrocyte content (including changed ones), increased protein concentration, neutrophilic pleocytosis in the cerebrospinal fluid. With the exception of cases of small focal parenchymal hemorrhages.
NSG is not very informative for small-point hemorrhages; massive hemorrhagic infarctions are visualized as asymmetric hyperechoic foci in the brain parenchyma. After 2-3 weeks, echo-negative cavities (pseudocysts, leukomalacia) form in their place. Possible contralateral displacement of the interhemispheric fissure and homolateral compression of the lateral ventricle.
CT scan shows areas of increased density in the brain parenchyma, varying in size and location, with concomitant deformation of the cerebrospinal fluid spaces.
MRI - change in the MR signal from foci of hemorrhage not in the acute stage.
DEG is asymmetric hypoperfusion in the cerebral arteries on the affected side.
I. B) Combined ischemic and hemorrhagic
CNS lesions (non-traumatic)
Occurs much more often
than all isolated forms of CNS damage discussed above
(occurs predominantly in premature infants)
a) Intrauterine hypoxia and asphyxia at birth. Premature infants with low body weight (1000-1500 g). Defects in the provision of primary resuscitation care, arterial hypotension, hypertension or fluctuations in systemic blood pressure. Coagulopathies, DIC syndrome.
B) The clinical picture depends on the leading type of damage to the central nervous system (ischemia or hemorrhage), its severity and location. There is significant variability in neurological symptoms and their dynamics. These types of damage are the most severe.
c) Metabolic disorders that are difficult to correct.
CSF - pressure is usually increased; morphological characteristics depend on the degree of hemorrhage into the cerebrospinal fluid spaces.
NSG, CT, MRI - various variants of deformation of the liquor-conducting system, foci of altered density, differing in intensity, predominantly periventricular localization.
DEG—fluctuation of cerebral blood flow; paralysis of the main arteries of the brain, decreased blood flow.
Diagnosis example:
“Combined (non-traumatic) ischemic-hemorrhagic damage to the central nervous system.”
In cases of diagnosing specific structural changes in the brain, combinations of codes corresponding to ischemic and hemorrhagic intracranial injuries are set (see Appendix).
Section II
Traumatic damage to the nervous system.
II. A) P 10 Intracranial birth injury
(Rupture of intracranial tissues and hemorrhage due to birth trauma)
R 10.8 Epidural hemorrhage
(occurs mainly in full-term,
with a frequency of about 2% among all intracranial hemorrhages).
a) Anomalies of childbirth: discrepancy between the birth canal and the size of the fetal head, anomalies of presentation, instrumental delivery.
b) Rapidly increasing intracranial hypertension in the first hours of life;
- Hyperexcitability;
- Convulsions;
- On the side of the hematoma, the pupil is sometimes dilated. Often combined with cephalhematoma.
c) Metabolic disorders in isolated epidural hematoma are not typical.
CSF is not informative.
NSG is not very informative (depends on the location and volume of the hematoma).
CT scan shows a ribbon-shaped, high-density formation between the dura mater and the covering bones of the skull. In some cases, the hematoma area has the shape of a “biconvex lens” adjacent to the integumentary bones of the skull.
DEG is not informative.
P 10.0 Subdural supratentorial hemorrhage
(True prevalence unknown, more common in full-term
over 4000 and post-term, in 40% of cases bilateral,)
a) See section “Epidural hemorrhage”
b) The following variants of clinical manifestations are found:
- Asymptomatic;
- Focal neurological disorders developing in the first 72 hours of life: hemiparesis (on the side opposite to the hematoma); deviation of the eyes in the direction opposite to hemiparesis (“eyes look” at the hematoma); dilation of the pupil on the side of the injury;
- Focal (focal) seizures
- Hypertension syndrome of varying severity or hyperexcitability.
c) Metabolic disorders in isolated subdural hematoma of convexital localization are not typical.
Transillumination of the skull is an accessible and informative diagnostic method. A limited focus of low luminescence above the hematoma is determined.
NSG - for small and flat subdural hematomas of convexital localization, is not very informative; for hemorrhages of significant size, there are signs of compression of the homolateral hemisphere and displacement of the median structures towards the side opposite to the focus.
CT and MRI are the most informative methods for diagnosing SDC of suprahemispheric localization. Hemorrhage is visualized as a “sickle-shaped” area of increased density adjacent to the calvarium.
DEG is a decrease in blood flow velocity in the middle cerebral artery on the side of the hematoma.
CSF changes are not very specific; lumbar puncture should be performed with great caution due to the high risk of herniation of the cerebellar tonsils into the foramen magnum, or the temporal lobe into the notch of the tentorium of the cerebellum.
P 10.4 Subdural subtentorial (infratentorial) hemorrhage
(They are rare, more often in full-term babies weighing
over 4000 and post-term)
a) Anomalies of childbirth: (discrepancy between the birth canal and the size of the fetal head, rigid birth canal, etc.), pathological variants of fetal presentation (usually breech), instrumental delivery.
b) Flow options:
- Catastrophic - from the first minutes and hours of life, signs of compression of the brain stem develop: progressive loss of cerebral activity - coma, opisthotonus posture, divergent strabismus, impaired pupillary reactions, floating movements of the eyeballs, fixed gaze. Progression of respiratory and cardiovascular disorders.
- Delayed or subacute-progressive - after a period of relative well-being (lasting from several hours or days, less often weeks), signs of intracranial hypertension (tension of the fontanelles, dehiscence of the nuchal suture, agitation, frequent tonic convulsions) and compression of the brain stem (respiratory and cardiovascular) increase. disorders, oculomotor, bulbar syndrome).
The most common outcome is death.
c) Metabolic disorders that are difficult to correct. Progressive decrease in blood pressure, bradyarrhythmia, anemia.
NSG is a deformation of the fourth ventricle; in some cases, zones of increased echogenicity are identified in the area of the structures of the posterior cranial fossa. Blood clots are detected in the cistern magna of the brain.
CT - allows you to detect extensive hematomas of the posterior cranial fossa, which are visualized as areas of increased density
MRI is the most informative for detecting hematomas of small volume in subacute cases.
CSF - lumbar puncture is not indicated due to the high risk of herniation of the cerebellar tonsils into the foramen magnum.
DEG is not informative.
R 10.2 Intraventricular hemorrhage, traumatic
(rarely encountered, mainly in full-term infants)
a) Prolonged labor, especially in combination with perinatal hypoxia, rapid rotation of the head, forced extraction of the fetus. Coagulopathies.
b) Manifestation - 1-2 days of life (in newborns with trauma and/or asphyxia) in newborns with unclear etiology (in 25%) - sometimes at 2-4 weeks of life.
- Hyperexcitability alternating with depression, convulsions (focal or multifocal), respiratory rhythm disturbances (secondary apnea).
- Progressive intracranial hypertension (vomiting, bulging fontanelle, dehiscence of cranial sutures).
- Hydrocephalus
c) There are no specific metabolic disorders.
NSG - ventriculomegaly, an uneven increase in the echogenicity of the choroid plexuses, deformation of their contours and an increase in size. Determination of echo-positive thrombi in the lumens of the ventricles.
CT, MRI - do not have obvious diagnostic advantages.
DEG is not informative
CSF - pressure is increased, blood admixture is determined in cases of blood penetration into the subarachnoid spaces, protein levels are increased, mixed pleocytosis (see above).
P 10.1 Parenchymal hemorrhage (hemorrhagic infarction)
(They are rare, more often in full-term babies weighing
over 4000 and post-term)
a) Anomalies of childbirth: (discrepancy between the birth canal and the size of the fetal head, rigid birth canal, etc.), pathological variants of fetal presentation, instrumental delivery. (Predisposing factors - hypoxia, foci of ischemia, coagulopathy, vascular malformations, tumors)
b) The clinical picture depends on the location and volume of hemorrhage.
Hemorrhages in the cerebral hemispheres - course:
- Asymptomatic;
- Increasing depression with a gradual loss of cerebral activity, transition to coma, often with focal symptoms (hemisyndromes, focal clonic convulsions),
- Intracranial hypertension (due to perifocal cerebral edema).
Intracerebellar hemorrhages - course
- Asymptomatic (with hemorrhage in the marginal parts of the cerebellar hemisphere);
- Increasing intracranial hypertension (tension of the fontanelles, dehiscence of the nuchal suture, agitation, frequent tonic convulsions).
- Compression of the brainstem (respiratory and cardiovascular disorders, oculomotor, bulbar syndrome) - with massive hemorrhages in the cerebellar hemispheres.
c) Metabolic disorders are not specific.
NSG - asymmetric hyperechoic areas of different size and location in the cerebral hemispheres; with a massive hematoma - signs of compression of the homolateral ventricle and displacement of the interhemispheric fissure. In the cerebellar hemispheres, hyperechoic foci are visualized (with significant intracerebellar hemorrhages).
CT and MRI are more informative for identifying parenchymal hematomas of various locations and sizes (especially subcortical and small ones).
DEG is not very informative in the acute period; later there are signs of cerebral hypoperfusion.
R 10.3 Traumatic subarachnoid hemorrhage
(rarely found, predominantly in full-term infants)
a) Anomalies of childbirth: (discrepancy between the birth canal and the size of the fetal head, rapid labor, rigid birth canal, etc.), pathological variants of fetal presentation, instrumental delivery, combined in 25% of cases with linear and depressed skull fractures. (Predisposing factors - hypoxia, coagulopathies, vascular malformations, tumors)
b) During the first 12 hours of life, depression of cerebral activity increases, up to coma. In some cases, a “waking” coma is observed: the eyes are wide open, a piercing cerebral scream, a decortication posture (flexion of the arms, extension of the legs).
- Hyperesthesia;
- Hyperexcitability;
- Rapidly growing external hydrocephalus (dehiscence of cranial sutures, bulging fontanelles);
- Generalized convulsions (occurring in the first hours of life).
c) Metabolic disorders are not specific. Posthemorrhagic anemia, blood pressure is reduced (vascular shock) in the first hours, subsequently uncontrolled systemic arterial hypertension.
NSG - possible increase in the echo density of the subcortical white matter on the side of the hemorrhage, expansion of the interhemispheric fissure and/or Sylvian fissure of the basal subarachnoid spaces. Subsequently, a progressive expansion of the convexital subarachnoid spaces is noted.
CT scan shows an increase in the density of the subarachnoid spaces, with their subsequent expansion.
MRI is not very informative in the acute period.
DEG is not very informative in the acute period; later there are signs of cerebral hypoperfusion.
CSF - high pressure, hemorrhagic cerebrospinal fluid, often reactive pleocytosis, elevated protein levels, by 3-6 days the macrophage reaction is pronounced.
II. B) Birth injury of the spinal cord
R 11.5 Hemorrhage into the spinal cord
(sprain, rupture, tear with or without spinal injury)
(rare, about 1% and mainly in full-term babies)
a) Anomalies of fetal presentation (gluteal and leg), incorrect implementation of obstetric aids (for example, excessive lateral traction or rotation of the body with a fixed head). Predisposing factors are hypoxia, coagulopathies, vascular malformations.
b) Three options for the clinical course:
- Catastrophic - stillbirth or death in the first hours after birth due to progressive respiratory and cardiovascular disorders. Observed at the craniospinal level of damage.
- Severe - accompanied by a picture of spinal shock lasting from several days to several weeks (adynamia, areflexia, atony), the abdomen is swollen, intestinal paresis, “paradoxical” diaphragmatic breathing, atony of the anal sphincter and bladder muscles, lack of pain sensitivity below the level of the lesion. Sometimes - Claude Bernard-Horner syndrome. Reflex reactions and sensitivity in the face and head are preserved.
The progression of respiratory failure often leads to death in the neonatal period. It is observed with damage to the middle and lower cervical, upper thoracic parts of the spinal cord.
- Moderately severe - the clinical picture of spinal shock is more short-lived, motor and reflex disorders are less pronounced.
c) Metabolic disorders characteristic of severe respiratory failure. Decreased systemic blood pressure, bradycardia, hypothermia.
NSG is not very informative.
CT, MRI - allow you to visualize the area and nature of the damage (MRI is more preferable).
ENMG - signs of denervation of skeletal muscles at the level of the lesion.
CSF - with hemorrhage, tears, ruptures - the cerebrospinal fluid is hemorrhagic, with ischemia - there may be an increase in protein levels.
II. B) P 14 Birth injury of the peripheral nervous system
Frequency of occurrence 0.1%, mainly in full-term infants
a) Incorrect implementation of obstetric care provided when it is difficult to remove the shoulders and head, or when the fetus’s arms are thrown back.
Traumatic injuries of the brachial plexus
b) P 14.0 Proximal (upper) Erb-Duchenne type
Flaccid paresis of the proximal arm: the arm is brought to the body, extended in all joints, the forearm is pronated, the hand is in palmar flexion, the head is tilted towards the sore shoulder, movements in the shoulder and elbow joints are limited, there is no reflex from the biceps brachii muscle, pain and tactile sensitivity reduced.
In approximately 5% of cases it is combined with paresis of the phrenic nerve.
P 14.1 Distal (lower) Dejerine-Klumpke type
Flaccid paresis of the distal arm: the arm is extended in all joints, lies along the body, pronated, the hand hangs passively. There are no spontaneous movements in the elbow and wrist joints, and movements in the fingers are limited. The grasping and palmo-oral reflexes on the affected side are not evoked. Often trophic disorders (edema, cyanosis, etc.). Sometimes this damage is accompanied by Claude Bernard-Horner syndrome on the affected side.
R 14.3 Total type (paresis of the brachial plexus).
Spontaneous movements in all parts of the arm are completely absent, diffuse muscle hypotonia, areflexia, impaired all types of sensitivity, trophic disorders. Often combined with Claude Bernard-Horner syndrome on the affected side.
c) There are no characteristic metabolic disorders.
NSG, CT, MRI, DEG are not informative.
CSF is not informative
ENMG - spontaneous bioelectrical activity in rest mode is absent; with active muscle effort, an interference type of curve is recorded with a reduced amplitude of oscillations in paretic muscles.
R 14.2 Traumatic injuries of the phrenic nerves
In 80-90% of cases it is combined with traumatic injuries of the brachial plexus
(total and proximal type), isolated paresis is extremely rare.
b) Unilateral paresis is clinically practically asymptomatic or with minimal manifestations of respiratory failure.
Bilateral paresis of the diaphragm leads to severe respiratory disorders from the first hours of life, which requires respiratory support in some cases.
c) Metabolic disorders characteristic of respiratory failure.
Ultrasound, chest X-ray - high standing and low mobility (relaxation) of the dome of the diaphragm on the affected side/sides.
R 11.3 Traumatic injury to the facial nerve
a) Abnormal presentation of the fetus, operative delivery - incorrect application of abdominal and exit obstetric forceps.
b) On the losing side:
- Lagophthalmos;
- Smoothness of the nasolabial fold;
- When screaming, the mouth is pulled to the healthy side, the search reflex is weakened.
c) NSG, CT, MRI are not informative.
ENMG - reveals a decrease in conductivity along the facial nerve.
R 14.8 Traumatic injuries to other peripheral nerves
(rare)
a) Anomalies of fetal presentation (breech and leg presentation), improper implementation of obstetric care. In the postnatal period - as a rule, of iatrogenic origin or of a secondary nature (inflammatory and traumatic changes in the bones and joints of the extremities).
b) Damage to the nerves of the extremities is clinically manifested by impaired movements and muscle tone in the corresponding areas of innervation: ulnar, radial, sciatic, obturator and tibial nerves.
c) If symptoms characteristic of damage to a particular peripheral nerve are identified, it is necessary to conduct a comprehensive examination to exclude traumatic and purulent-inflammatory processes in the bones, joints and soft tissues.
Continued >>
<< Back
Restoration of vital functions
The next stage involves the restoration of vital functions. Thus, it is necessary to restore blood circulation, breathing and normal heart function. Further therapy is aimed at restoring all previously lost functions. For these purposes, neurometabolites are prescribed along with nootropics, vascular drugs, neuroprotectors and antioxidants.
Symptomatic therapy is aimed at eliminating the main manifestation of the consequences of anoxia. In case of severe headache, analgesics are used, and against the background of epileptic seizures, anticonvulsants are required, and so on.