Cortexin is a drug product, which means that, like many other drugs, it has certain side effects.
First, we need to understand what we mean by side effects.
After using the medicine, a certain effect develops. This occurs due to interaction with certain receptors and changes in the structure of some components of our body (for example, blood vessels).
Most diseases affect a specific location in the body, such as the brain or heart. At the same time, making the tablet work exactly where it “hurts” is a very difficult task.
Firstly, even with targeted administration of the drug to the place where it is most needed, the active substance will spread to neighboring areas due to diffusion through tissues.
Secondly, due to the abundant blood supply to the whole body, the injected drug will be distributed through the bloodstream to other areas of the body.
Thirdly, from a chemical point of view, synthesizing a drug that would interact with only one specific receptor is an extremely difficult task.
Therefore, often the medicine acts not only where it is needed, but also in other places, causing additional effects. It is these effects that are not necessary at the moment that are called side effects.
It is worth noting that the incidence of side effects is usually low. If medical prescriptions are strictly followed (dosage, frequency of administration, connection of intake with food intake, etc.), unwanted reactions occur quite rarely. There are no data on specific side effects of Cortexin. None of the clinical and laboratory studies recorded side effects or undesirable effects. This is ensured by the high tissue specificity of the drug, that is, the ability to “hit” exactly where it is needed.
However, Cortexin is a lyophilisate of the cerebral cortex of cattle, that is, a drug of animal nature. This means that there is a risk of developing allergic reactions.
An allergic reaction is an excessive reaction of the body to a foreign, harmless substance. Our body can perceive a substance from the outside as a pathogenic microorganism, a dangerous virus or a parasite. Depending on the state of the body and the method of entry of the allergen, the reaction can occur in different ways:
- Allergic rhinitis;
- Allergic conjunctivitis;
- Food allergies;
- Bronchospasm;
- Bronchial asthma;
- Eczema, urticaria, contact dermatitis, atopic dermatitis;
- Generalized forms of allergic reactions (Quincke's edema, multimorphic exudative erythema, Lyell's syndrome, Stevens-Johnson syndrome, anaphylactic shock);
To reduce the possibility of developing allergic reactions, during the production of Cortexin, chemical, thermal and vacuum treatment of the original substrate occurs.
Pharmacological properties of the drug Cortexin
A drug of a polypeptide nature that has a tissue-specific effect on the cerebral cortex. It has cerebroprotective, nootropic and anticonvulsant effects, reduces the toxic effects of neurotropic substances, improves the learning process, memorization, stimulates reparative processes in the brain, accelerates the recovery of brain function after stressors. The mechanism of action of Cortexin is associated with its metabolic activity: the drug regulates the ratio of inhibitory and excitatory amino acids in the central nervous system, the level of serotonin and dopamine, has a GABAergic effect, has antioxidant activity and the ability to restore the bioelectrical activity of the brain. Cortexin is a balanced, optimal combination of a complex of neuropeptides and L-amino acids with an average half-life of up to 30 minutes, which does not allow determining the absorption, distribution and excretion of peptide residues.
What side effects may occur in children?
The child’s body is immature in many respects, including the state of the immune system. The development of the following allergic reactions is typical for children:
- Atopic dermatitis (the skin turns red, becomes moist, and cracks may appear);
- Allergic conjunctivitis (inflammation of the mucous membrane of the eyes) and allergic rhinitis (runny nose);
- Food allergies (nausea, vomiting, bloating, belching, bowel problems such as constipation or diarrhea);
Allergic reactions develop faster in children than in adults. The severity of these reactions varies - from minor peeling of the skin to generalized swelling of the entire body. Often, the risk of side effects depends on the child’s pre-existing condition and the presence or absence of diseases.
When Cortexin was administered to children, no adverse reactions were observed.
Chronic fatigue syndrome and its correction with Cortexin
About the article
6518
0
Regular issues of "RMZh" No. 16 dated July 21, 2010 p. 1004
Category: Neurology
Author: Tsygan V.N.
For quotation:
Gypsy V.N. Chronic fatigue syndrome and its correction with Cortexin. RMJ. 2010;16:1004.
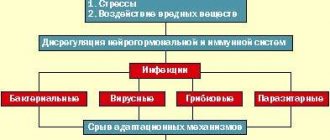
Increased fatigue, general weakness, constant fatigue and malaise are the most common complaints expressed by patients with various diseases. These symptoms are common to nosological forms with different pathogenesis. Healthy people may also complain of increased fatigue and long-term (chronic) fatigue. However, their condition usually improves significantly after adequate rest and simple rehabilitation measures. Chronic fatigue syndrome (CFS) differs from a transient state of weakness in healthy people and in patients with various diseases in the initial stage and in the convalescent stage in terms of the duration and severity of psychosomatic disorders. The clinical manifestations of CFS are comparable to the classical concepts of the disease as an independent nosological unit. In addition to increased fatigue and general weakness, which significantly reduce performance, patients often complain of headaches, sleep disturbances, deterioration of memory and concentration, mental depression, pain in muscles and joints, pain in the throat and cervical lymph nodes, and constant low-grade fever.
CFS was first classified as a disease in the statistical reports of the Center for Disease Control (Atlanta, USA) in 1988. Since then, doctors and scientists around the world have been working to unravel this phenomenon - another disease of civilization, usually affecting active and purposeful people. Specialized scientific centers have been created in the USA, Great Britain and Australia to study this problem. In Russia, CFS began to be dealt with in recent years, although conditions similar to this syndrome, under various names - “neurasthenic syndrome”, “asthenic syndrome”, “neurocirculatory dystonia”, “myalgic encephalomyelitis” - were known to doctors back in the 19th–20th centuries . Despite the active debate, there is still no consensus on the etiology and pathogenesis of this syndrome [1,5]. In recent years, there has been a tendency to increase the number of such patients. Today, about 17 million people worldwide suffer from CFS. The reason for the increased incidence of CFS development may be the deterioration of the environmental situation, increased stress loads, the spread of viral infections, and an increase in the number of immunologically compromised individuals. Currently, various drugs are used to treat patients with CFS [1]. However, the variety of clinical manifestations and insufficient knowledge of pathogenetic mechanisms determine the low effectiveness of existing treatment methods. Certain hopes are placed on the use of polypeptide nootropic drugs, because they effectively restore impaired metabolism and integrative functions of the brain. One of the most popular drugs in this group is Cortexin. It is a complex of polypeptides with a molecular weight from 1000 to 10,000 daltons. According to the results of previous studies, the drug has a cerebroprotective effect on the cerebral cortex [2,4]. To evaluate the effectiveness of Cortexin for CFS, we conducted a study. Materials and methods The study included 78 patients with CFS. The following criteria for diagnosing CFS were used: 1) the presence of chronic fatigue, which is defined as clinically established, unexplained, constant or intermittent chronic fatigue of a new type (not previously encountered during life), not associated with physical or mental stress, not relieved by rest and leading to a significant drop in previously achieved levels of professional, educational, social or personal activity; 2) the simultaneous presence of four or more of the following symptoms (all symptoms can exist constantly or recur for 6 or more months): • headaches that differ in nature from those previously observed; • muscle pain; • pain in several joints in the absence of itching and redness; • unrefreshing sleep; • discomfort after physical or neuropsychic stress lasting more than 24 hours; • impairments of short-term memory or concentration that significantly reduce the level of professional, educational or other social and personal activity; • signs of inflammation of the mucous membrane of the throat; • tenderness of the cervical or axillary lymph nodes. Cases of idiopathic chronic fatigue were defined as clinically diagnosed chronic fatigue that did not meet the criteria for CFS. The examined patients were divided into two groups: I (main) – 56 patients, II (control) – 22 patients. The duration of the period from the onset of CFS ranged from 6 months to 3 years. The average age of the examined patients was 31.7±8.2 years (from 19 to 46 years). The basis of treatment was physiotherapeutic procedures, non-steroidal anti-inflammatory drugs, sedatives, herbal adaptogens, multivitamins, and symptomatic therapy. Patients of the main group received the peptide drug Cortexin according to the following regimen: daily intramuscularly for 10 days, 1 bottle (10 mg) of the drug Cortexin, dissolved in 2 ml of water for injection or isotonic sodium chloride solution. If necessary, the course was repeated after 1, 3, 6 months. To objectify the therapeutic effect of the drug and assess its effect on impaired functions in patients, in addition to clinical neurological examination, data from psychophysiological, electrophysiological, Doppler and immunological studies were used, which were carried out before and after the course of treatment. The results of clinical and functional research methods after the course of treatment were assessed as good, satisfactory and unsatisfactory. Results and discussion Typically, the patient exhibited several symptoms. The leading symptom was considered to be the one whose clinical manifestations, both subjective and objective, were most pronounced and dominant at the time of the examination. Most often, patients complained of increased fatigue, headaches, periodic dizziness, decreased memory and attention, and irritability. Clinically good results under treatment with Cortexin were obtained in 43 (77%) patients. Moreover, after treatment, the patients did not complain or noted a significant decrease in their intensity; During an objective examination, a clear decrease in the manifestations of CFS was noted. A satisfactory result was obtained in 11 (20%) patients. At the same time, the examined patients partially retained their complaints, but the degree of their severity noticeably decreased; During an objective examination, moderate positive dynamics of pathological manifestations were noted. An unsatisfactory result was observed in 2 (4%) patients. Their complaints remained, the degree of their severity remained virtually unchanged; During an objective examination, the absence of positive dynamics was noted. In patients of group I, vegetative and asthenic manifestations were relieved in more than 60% of cases, and in 55% of cases, neurological symptoms significantly regressed. The most pronounced effect of Cortexin was observed in patients with asthenic syndrome and cognitive impairment. This is apparently explained by the fact that the drug improves energy metabolism, stimulates redox processes in the brain, and helps normalize the metabolism of neurotransmitters. A particularly pronounced effect from the use of Cortexin was identified based on the results of assessing psychophysiological parameters. A study of a simple visual motor reaction, which allows us to determine the level of performance of patients, showed differences (p<0.05) between the initial level of performance and the level achieved as a result of therapy. If before treatment with Cortexin, performance was significantly reduced in 33.7% of patients and moderately reduced in 36.5%, then after treatment these figures were 19 and 12%, respectively. Reliable (p<0.05) indicators were also obtained when studying the state of anxiety on the Spielberger-Khanin self-esteem scale. After treatment with Cortexin, most patients managed to achieve a normal level of anxiety. When performing a proofreading test, differences (p<0.05) were revealed in the number of characters viewed in patients of the main and control groups. At the same time, after completing the course of treatment with Cortexin, the performance of the proofreading test improved (p<0.01), the number of characters looked up noticeably increased (p<0.01) and the number of errors decreased. Thus, the use of Cortexin significantly improved the function of attention in the absence of signs of a rapid decline in attention stability. A study of short-term memory using the method of memorizing 10 words revealed a significant decrease in the volume of voluntary attention. In 63% of patients, instability of the memorization processes was revealed, which was manifested in fluctuations in the number of words retained in memory when performing a task and the need for a greater number of repetitions of words compared to healthy people. After completion of Cortexin treatment, a 10-word memorization test showed a significant increase in short-term memory in almost all subjects. It should be noted that before the start of treatment, 9 (16%) people were not able to complete the task due to a sharp increase in fatigue. To assess the effect of Cortexin treatment on the functional activity of the brain, a visual analysis of the EEG was carried out with the distribution of electroencephalograms by type and the calculation of the alpha index [6]. Thus, before treatment, EEG types III, IV and V predominated in 48.4% of patients according to E.A. Zhirmunskaya. (1991). After a course of treatment with Cortexin, zonal differences in the alpha rhythm were restored, the severity of irritative processes was weakened, and in two cases, paroxysmal activity disappeared. As a result of the clear positive dynamics of the visual EEG pattern, the structure of EEG types also changed, which was expressed in an increase in the number of normal and conditionally pathological types while the number of pathological EEG types decreased by almost half. A study of hemodynamics after the course of treatment showed that an increase in blood flow was noted in 40% of patients, in 52% of patients the blood flow did not change significantly, and in 8% there was a slight decrease. In 27.6% of people in this group, the asymmetry coefficient normalized. The clinical effect was accompanied by normalization of the content of T lymphocytes and their mitogenic response, redistribution of cells into the CD8+ population with an increase in the number of antigen-dependent cytotoxic effector lymphocytes and natural killer cells (Fig. 1). The B-link of immunity was characterized by a decrease in the number of B-cells, while the number of differentiated cells expressing surface immunoglobulins G and blood IgG content increased, while the amount of IgM decreased (Fig. 2). In both groups of patients, the improvement in well-being and regression of CFS symptoms during treatment coincided with the positive dynamics of psychophysiological, electrophysiological, hemodynamic and immunological parameters. Conclusion The clinical study conducted indicates that Cortexin has not only nootropic and neuroprotective effects, but also a positive effect on the immune status. It can be assumed that CFS includes psychosomatic diseases with a pronounced immunopathological component. When developing treatment tactics for CFS, the drug of choice is the domestic drug Cortexin, which has nootropic and immunomodulatory properties.
References 1. Artsimovich N.G., Galushina T.S. Chronic fatigue syndrome. – M.: Scientific. world, 2002. – 220 p. 2. Batysheva T.T., Boyko A.N., Dyakonov M.M. and others. Neuroprotection in the treatment of chronic cerebrovascular insufficiency. - Vestn. Ross. Military-med. acad., 2007. – No. 1(17). – pp. 11–18. 3. Zhirmunskaya E. A. Clinical electroencephalography. – M.: Maby, 1991. – 77 p. 4. Peptide neuroprotection / ed. MM. Dyakonova, A.A. Kamensky. – SPb.: Science. 2009. – 256 p. 5. Chronic fatigue syndrome: diagnosis and treatment / ed. Yu.V. Lobzin. – St. Petersburg: SpetsLit, 2005. – 79 p. 6. Electroencephalography / V.N. Gypsy, M.M. Bogoslovsky, A.V. Mirolyubov // ed. MM. Dyakonova. – St. Petersburg: Nauka, 2008. – 192 p.
Content is licensed under a Creative Commons Attribution 4.0 International License.
Share the article on social networks
Recommend the article to your colleagues