Experts differ on the causes of this disease. Neurologists are dominated by two points of view regarding the circumstances that precede the onset of pathology.
- Hereditary factor (benign epilepsy of infancy occurs precisely due to the genetic predisposition of the child’s body).
- Impaired development of the cerebral cortex (lag in the development of nerve cells) - recent research inclines neurologists to this position, which is indirectly confirmed by the cessation of the disease upon reaching adolescence.
What scientists agree on is the reason for the recovery of children upon reaching puberty: foci of epilepsy disappear when the excitability of nervous tissues decreases, which happens as they mature.
Causes of benign rolandic epilepsy
The exact causes of the disease are unknown. However, there is a hypothesis that confirms the hereditary nature of the pathology. The disease may occur due to a pathological disorder in the maturation of the cerebral cortex in the central temporal region.
As for benign epilepsy, its dependence on the patient’s age has been proven, since it is often diagnosed in children. The pathology may be caused by increased excitability of the children's brain. This can happen for a variety of reasons:
- predominance of excitatory neurotransmitters;
- structural and functional features of epileptogenic areas (hippocampus, neocortex, amygdala);
- low GABA content;
- increased number of excitatory reentrant synapses;
- immaturity of GABA receptors.
As the child's brain matures, its excitability decreases, and foci of pathology gradually disappear. Usually, after children reach puberty, the number of seizures decreases until they disappear completely.
Causes
The etiology has not yet been definitively established. 20–60% of patients have close blood relatives who have the same disease, but there is no direct autosomal dominant or autosomal recessive transmission. Taking this into account, researchers believe that the pathology is inherited not by one, but by two different genes.
The mechanism of development has not yet been precisely clarified. The most popular generally accepted theory is that seizures are caused by impaired maturation of the temporal cortex and increased excitability of cerebral structures in children. As we grow older, the excitability of the brain decreases, the lesions cease to exhibit epileptogenic activity, epileptic attacks become more rare and then disappear.
Symptoms of benign rolandic epilepsy
With rolandic epilepsy, patients periodically experience seizures that are moderate in nature. In this case, the attack occurs without the patient losing consciousness. Often, the patient experiences a sensory aura in front of him, which is characterized by sensations such as tingling, tingling, numbness in the gums, face, throat, tongue and lips. Then strong tonic and muscle contractions occur.
If a patient experiences a hemifacial attack, they experience muscle spasms on one side of the face. During a pharyngeal attack, unilateral convulsions of the tongue, lips, larynx and pharynx occur, which are inevitably accompanied by speech impairment. Due to convulsive contractions of the muscles of the larynx, guttural sounds appear that resemble gurgling or gargling.
Patients' attacks occur mainly at night. They are often observed during the period of falling asleep or waking up. In rare cases, both daytime and nighttime attacks are observed. In 20% of all cases of the disease, brachiofacial seizures occur, which are characterized by the spread of facial spasms to the homolateral arm. Patients may also experience more severe forms of seizures, in which spasms involve all the muscles of the body. Typically, such extensive convulsions cause the patient to lose consciousness.
Symptoms and signs of epilepsy
Epilepsy is classified according to the location of the electrical discharge. This may be a separate area in the brain, or the discharge covers two hemispheres at once (generalized seizures).
? When the entire brain is involved at once, it is customary to speak of primary generalized seizures:
- Tonic-clonic seizures. This is epilepsy in its “classical” presentation: seizures with loss of consciousness, switching off, falling, convulsions, rolling out eyes, biting the tongue, etc. A person suffering from epilepsy, after a generalized attack, does not remember what is happening and feels very tired and can fall asleep.
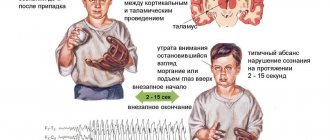
- Absence seizures. Short blackouts of consciousness for 10 seconds to 2 minutes without convulsions. After an attack, the condition remains unchanged; absence seizures are often mistaken for the child’s absent-mindedness.
? When nerve cells are damaged in one area, the seizure focus is located in one zone, but then it can spread to both hemispheres.
These are focal (partial) seizures of epilepsy. They are the most common among all cases of epilepsy and are divided into:
Simple - attacks without switching off or changing consciousness. Manifestations of simple focal epileptic seizures are different:
- motor signs: twitching, tics, involuntary movements in one part of the body, “marching”, other uncontrolled body movements and facial expressions
- changes in sensory perception: hallucinations, seemingly foreign sounds, strange taste in the mouth, dizziness
- sweating, redness of the face, changes in pupils
- vocalizations: the person may shout different words uncontrollably
Complex - consciousness changes or turns off, a person may not remember what happened. Complex focal seizures of epilepsy can develop into generalized ones.
They are characterized by altered behavior:
- motor automatisms
- motor disorders
- disturbances of consciousness
- complex hallucinations
- anger, phobias
- dementia (a state of deja vu, an event already seen or experienced), etc.
? Secondary generalized epileptic seizures begin in one area and subsequently spread to both hemispheres. They begin in the form of convulsions or disturbances of consciousness and turn into a severe epileptic seizure involving all muscle groups.
Among the types of epilepsy there are different forms:
- Benign epilepsy (familial, idiopathic, myoclonic seizures). Goes away on its own, does not transform into other forms;
- Febrile convulsions (with an increase in temperature, may be an isolated case of an epileptic seizure);
- Absence epilepsy – onset in childhood, characterized by loss of consciousness;
- Neonatal seizures (with onset 1-12 months) - West syndrome, Ottahar syndrome - severe pathology, entails disability, most often develops into another form of epilepsy);
- Generalized epilepsy with tonic-clonic seizures and others.
Course of benign rolandic epilepsy
Benign rolandic epilepsy is characterized by short-term attacks, the duration of which is about 2-3 minutes. Typically, such attacks are isolated and occur several times a year. At the beginning of the disease, epileptic seizures may occur frequently, but over time they occur much less frequently. It is important to note that this type of epilepsy will not be dangerous for children, since it does not provoke a delay in their psychomotor development and mental retardation. However, in rare cases, neurological damage occurs.
General information
If we consider all benign focal epilepsies, then rolandic is the most common among pediatric patients. According to statistics, it accounts for 15% of the number of diseases in children under 15 years of age and approximately 20% of the manifestation of seizures in them.
For every 100,000 children, the disease will manifest itself in at least 21 people. The period in which this disorder may appear is approximately 12 years: from 2 to 14. Obviously, the main period in which about 85% of people get sick is between the ages of 4 and 10.
A distinctive feature of the disease is the complete disappearance of pain, convulsions and seizures by the age of 15-18, as well as the complete absence of any dangerous effects on psychophysical health.
Diagnosis of benign rolandic epilepsy
The disease can be most accurately diagnosed using electroencephalography (EEG). This technique is used to study the functional activity of the brain and identify pathological disorders in it. However, it is worth noting that in some patients, changes in the EEG may only be noticeable during sleep. That is why the doctor may additionally prescribe an overnight EEG or polysomnography.
A neurologist can make a diagnosis if the following pathology is detected: identification of high-amplitude sharp waves or peaks that are localized in the central temporal leads. At the same time, the functional activity of the brain is normal. The EEG results in this case show that the peaks or sharp waves are immediately followed by slow waves, which together constitute the “Rolandic complex”. This symptom can be either bilateral or unilateral.
Diagnostics
Rolandic epilepsy is supported by the typical age of onset of symptoms, the presence of relatives with similar manifestations, rare attacks and their predominantly nocturnal nature. The main objective research method to confirm the diagnosis is electroencephalography. It should be taken into account that when recording during the interictal period, changes are often absent. To obtain reliable data, polysomnography or EEG video monitoring is required.
The “Rolandic complex” on encephalograms lasts up to 30 milliseconds and is represented by sharp peaks followed by slow waves in the temporal leads. Most often detected on the side contralateral to the area where seizures occur, sometimes detected on both sides. A distinctive feature is the instability of complexes during several successive recordings separated by a time interval.
An important part of diagnostic measures is to exclude other diseases that can cause similar manifestations. The disease is differentiated from symptomatic focal epilepsy due to encephalitis, meningoencephalitis, brain abscess, head injury, and intracerebral neoplasms. The Rolandic variant is supported by the absence of neurological deficits and behavioral disorders, completely intact intelligence, and a typical EEG pattern. In doubtful cases, an MRI is additionally prescribed.
Serious difficulties can arise in differentiating the rolandic and nocturnal types of epilepsy, which is associated with the difficulty of distinguishing between speech and consciousness disorders during nocturnal paroxysms. When making a final diagnosis, they rely on electroencephalogram data.
Differential diagnosis of benign rolandic epilepsy
Symptoms of benign rolandic epilepsy are similar to those of other neurological pathologies. First of all, this disease should be differentiated from other types of epilepsy provoked by traumatic brain injuries, neoplasms, inflammatory lesions of the brain due to encephalitis, meningitis, and abscess. The main distinguishing feature of the pathology is the absence of behavioral disorders and serious changes in neurological status in the clinical picture, as well as normal EEG activity and complete preservation of intelligence. A more accurate differential diagnosis can be made using MRI of the brain.
Neurologists note that it is most difficult to differentiate benign rolandic epilepsy from nocturnal epilepsy. In this case, speech disorders that are quite typical for pathology are often interpreted as disorders of consciousness. Therefore, to make a correct and accurate diagnosis, it is extremely important to conduct an EEG. If the patient has temporal lobe epilepsy or frontal lobe epilepsy, the results will show the presence of focal changes in brain activity.
Neurologists often have difficulty differentiating benign rolandic epilepsy and pseudolennox syndrome. The most pronounced signs in which a patient is diagnosed with pseudolennox syndrome will be: a complex of atypical absence seizures with typical rolandic epileptic seizures, the presence of intellectual-mnestic disorders, detection of slow complexes or diffuse peak activity during EEG.
Rolandic epilepsy
K.Yu. Mukhin, P.A. Temin, E.A. Rykova
Luigio Rolando (1773-1831), Italian physician and anatomist, gave the classic topographical description of the central sulcus of the cerebral cortex. In idiopathic partial epilepsy of childhood, characterized by short hemifacial motor nocturnal seizures, often with a preceding somatosensory aura and typical EEG changes (polyphasic spikes localized in the central and medial temporal regions) [14], the epileptogenic zone is the lower parts of the Rolandic sulcus. The study of this type of idiopathic partial epilepsy began more than 40 years ago. Initially, specific EEG changes were described in a certain group of children. In 1952, H. Gastaut [25] first drew attention to special epileptic EEG changes in some children: polyphasic spikes with strict localization in the perirolandic region. The first clinical description of the symptoms of epilepsy with rolandic spikes (1958) belongs to R.Nayras and M.Beaussart [40]. Subsequently, E. Gibbs and F. Gibbs [27], S. Lombroso [36], R. Loisea et al. [35] conducted a clinical analysis of symptoms in epilepsy with rolandic spikes and proposed calling this form of epilepsy rolandic. The authors noted her favorable prognosis and the absence of symptoms of organic damage to the central nervous system.
Frequency
Rolandic epilepsy (RE) is one of the most common forms of childhood epilepsy. Its prevalence is 21 per 100 thousand healthy children [29]. The frequency of EC among all forms of epilepsy with onset before 13 years of age varies, according to various sources, from 11.5% [15] to 25% [5]. Of the 360 children with epilepsy observed by R. Lerman [34], 14.4% were diagnosed with rolandic epilepsy. For comparison, we note that one of the most common forms of childhood epilepsy - pycnolepsy - was diagnosed in only 10.5% of patients. It is possible that the true incidence of rolandic epilepsy in the population is much higher, since many patients experience single nocturnal attacks, which often go unnoticed by parents and doctors [34].
Most authors note the predominance of boys among patients with rolandic epilepsy [3]. The average ratio of boys to girls is 6:4 [11]. Among the 100 points observed by R. Lerman [34], there were 62 boys and 38 girls.
Clinic
The onset of Rolandic epilepsy varies in the age range from 2 to 14 years. In 83% of cases, EC debuts at the age of 4–10 years[5]. In the vast majority of patients, attacks begin between the 5th and 10th year of life, with a maximum at the age of 9 years [9]. The average age of onset of attacks is 9.9 years [33]. The onset of the disease before the age of 2 years is observed in no more than 8% of cases [15]. There are isolated descriptions of the onset of EC in the 1st year of life [17], but the classification of these cases as EC is debated [21]. The onset of the disease after 11 years is also rare, and after 14 years such cases are not observed [9].
The clinical symptoms of seizures in rolandic epilepsy are usually typical. Simple partial (motor, sensory, autonomic), complex partial (motor) and secondarily generalized seizures are observed. The most typical are simple partial motor and/or sensory paroxysms [2].
Simple partial seizures constitute the “core” of rolandic epilepsy and are observed in 70 - 80% of patients. The most typical onset of an attack is with a somatosensory aura: a sensation of tingling, numbness, “passage of electric current” unilaterally in the pharynx, tongue, and gums. Following the aura, a partial attack develops. The following options are possible: hemifacial attacks; unilateral tonic, clonic or tonic-clonic spasms of facial muscles; pharyngeal attacks; unilateral convulsions of the lip, tongue, pharynx, larynx, often combined with anarthria and hypersalivation. Hemifacial seizures occur in 37% of patients, pharyngooral - in 53% [91. Often in their sleep, patients make peculiar throat sounds such as “gurgling”, “grunting”, “gargling”. The true frequency of somatosensory attacks (buccal paresthesia according to the terminology of J. Aicardi [5]) is difficult to determine, since children, especially young ones, find it difficult to describe their sensations during attacks.
In 20% of patients, seizures can spread from the facial muscles to the homolateral arm (brachiofacial seizures) and in approximately 8% of cases involve the leg (unilateral seizures). As the disease progresses, attacks may change direction. Secondary generalized seizures are observed in 20-25% of patients with rolandic epilepsy. They are most typical for younger children, and the occurrence of generalized attacks is “rigidly” timed to sleep. The presence of only generalized convulsive seizures in patients for a long time is not typical for RE, as is the presence of absence seizures, psychomotor seizures, and seizures with mental symptoms [5].
The duration of attacks in rolandic epilepsy is usually short [5], from a few seconds to 2-3 minutes. In 11% [16] - 22% [5] of patients, the duration of paroxysms exceeds 10-15 minutes, in 16% [5] severe prolonged attacks ending in post-attack hemiparesis (Todd's palsy) are noted. The frequency of attacks in EC is usually low - on average 2 per year [5]. In the first 1-2 years from the onset of the disease, attacks may be more frequent, but over time they occur less and less often. After 13-16 years, complete disappearance of attacks is possible. 10-40% of children experience a single attack during the entire illness, even if they do not receive anticonvulsants [41]. Weekly or more frequent, daily attacks are observed only in 6% [35] - 20% [34] of cases. Serial attacks and repeated attacks within one night are rare and were observed in 3% of cases described by T. Deonna et al. [17].
Rare publications [15,16,23,34] report the development of status epilepticus in EC. Often it is about 11% in RE [16]. A detailed description of status epilepticus in patients with EC is presented in the work of N. Fejerman and A.Di Blasi [23]. The authors observed 2 8-year-old boys with the onset of EC at 3 years of age, who developed a long-term status of hemifacial seizures. Consciousness was preserved, but anarthria and severe drooling were noted. Oral administration of anticonvulsants in high doses, as well as parenteral administration of diazepam or lorazepam, had no effect on status epilepticus. Only the administration of hormonal drugs (dexamethasone) completely stopped the seizures. However, the attribution of these cases to Rolandic epilepsy is questionable. One of these patients had typical absence seizures, the other had Jacksonian march attacks; Both had seizures that were resistant to anticonvulsants. It is possible that these cases represent an atypical form of EC. E.Roulet et al. [42] described status epilepticus in a 6-year-old boy with rolandic epilepsy. Its only manifestation was prolonged drooling in combination with orolinguomotor dyspraxia. Spike-wave complexes, including Rolandic complexes, were recorded on the EEG during salivation. When the dose of carbamazepine was increased, the drooling stopped. According to the authors, prolonged permanent salivation in patients with rolandic epilepsy may be the only symptom of status epilepticus.
RE paroxysms are associated with the sleep-wake rhythm. The most typical attacks are nocturnal, occurring primarily when falling asleep and waking up. During nocturnal attacks, paroxysms occur during awakening (35%), less often - in the middle of the night (25%) and when falling asleep (20%). In 25-30% of patients, attacks are observed both during sleep and while awake [5]. Only in 5-25% of patients they occur exclusively during wakefulness [5,34]. There is some difference in the pattern of attacks depending on the sleep-wake rhythm. Daytime attacks are almost always simple partial, often hemifacial, short, nocturnal, usually more severe, prolonged, usually unilateral or secondarily generalized. Assessing consciousness in patients with nocturnal seizures is difficult; the phenomenon of anarthria often simulates the clinic of switching off consciousness. Complex partial seizures in rolandic epilepsy are rare and account for only about 5% of all cases [15]. It is possible that during an attack in some patients there is a “fluctuation” in the level of impairment of knowledge.
J.Aicatdi and J.Chevrie [4] identified a group of patients with a peculiar epileptic syndrome, the clinical symptoms of which were similar to Rolandic epilepsy. The patients had simple partial hemifacial and hemiclonic seizures, however, in combination with myoclonic-astatic, atonic seizures and, in some cases, absence seizures. This group of observations is classified as an atypical variant of EC. Its frequency, according to T. Deonna et al. [16], accounts for 5% of all patients with EC. The taxonomic position of this syndrome is controversial. According to J. Aicatdi and J. Chevrie [5], this form of epilepsy is a nosologically independent syndrome with a clearly defined clinical picture, EEG changes and prognosis. Other authors suggest the presence of an atypical variant within EC [17]. A detailed description of this syndrome is presented by T. Deonna et al. [16] on the example of 6 patients. Among the examined patients, boys predominated. The attacks began in the age range from 2 to 7 years in neurologically normal children with intact intelligence. Clinical symptoms included various types of seizures. Characteristic were nocturnal simple partial seizures, similar to those in RE, myoclonic-astatic and atonic seizures. A number of patients experienced atypical absence seizures. The attacks in all cases were frequent, daily and led to repeated falls of patients and severe bruises. On the EEG, typical rolandic spikes were combined with slow peak-wave complexes characteristic of Lennox-Gastaut syndrome. There was an increase in epileptic activity in the slow-wave sleep phase.
The clinical symptoms of atypical rolandic epilepsy bear the features of a number of epileptic syndromes: RE (simple partial nocturnal seizures combined with typical rolandic spikes), Lennox-Gastaut syndrome (frequent atonic-astatic seizures, atypical absences in combination with slow peak-wave complexes) and myoclonic epilepsy - astatic attacks described by N. Doose [19]. However, the cardinal difference between atypical rolandic epilepsy and Lennox-Gastaut syndrome and epilepsy with myoclonic-astatic seizures is the absence of intellectual impairment in patients and a good prognosis. R. Loisea et al. [35] conducted a follow-up study of 13 patients with atypical EC for at least 9 years. After varying periods of time, complete remission was achieved in all cases, while the majority of patients (10) did not use regular antiepileptic therapy. The authors noted neither mental retardation nor serious behavioral disorders both during the most active period of the disease and during the period of remission, which brings these cases closer to typical EC. According to the authors, “aggressive” antiepileptic therapy should be avoided in this syndrome due to the good prognosis.
O. Dulac et al. [21] analyzed cases of partial epilepsy with early onset - between 8 days and 3 years of life. Among 442 patients, 17 with partial epilepsy with early onset were identified. The attacks were characterized as simple partial with frequent secondary generalization. According to the authors, these cases are a transitional form between benign epilepsy of newborns and EC. In contrast to the two indicated syndromes, the prognosis was unfavorable; the attacks were not amenable to drug therapy. Subsequent studies will show whether cases of partial epilepsy with onset in the first 3 years of life are a nosologically independent syndrome or another “atypical variant” of EC.
Neurological status
The neurological status of children suffering from rolandic epilepsy has been studied in sufficient detail. Rolandic epilepsy refers to idiopathic forms of epilepsy in which there are no signs of organic brain damage, which is confirmed by most publications. Moreover, according to A. Beaumanoir et al. [8] the presence of such symptoms contradicts the criteria for the diagnosis of rolandic epilepsy. However, in recent years, thanks to the introduction into clinical practice of highly effective neuroradiological research methods (computed tomography, nuclear magnetic resonance, positron emission tomography), isolated reports have appeared on the detection of structural changes in the brain in patients with EC.
Of the 100 patients observed by P. Lerman and S. Kivity [33], 4 had symptoms of organic damage to the central nervous system: cerebral palsy (hemiparetic and tetraplegic forms), microcephaly, moderate mental retardation. In all these cases, the diagnosis of EC was beyond doubt and was confirmed both clinically and neurophysiologically. Pneumoencephalography did not reveal any abnormalities in these patients. S. Blom and J. Heijbet [11] found central hemiparesis in patients in 3 (7.5%) of 40 cases of EC. E.Roulet et al. [42] noted manifestations of orolinguomotor dyspraxia in a patient with EC. Symptoms such as microcephaly, strabismus, cerebellar insufficiency, delayed mental and speech development were noted in a number of patients with EC by R. Santanelli et al. [43]. Hyperactivity syndrome was found in 16.7% of patients with EC [34].
Genetics
R. Bray and W. Wiser [13] were the first to note the presence of familial cases of rolandic epilepsy and suggested an autosomal dominant model of inheritance with incomplete penetrance and age dependence. In recent decades, numerous clinical and genealogical studies have been conducted in rolandic epilepsy, confirming the validity of this concept. According to generalized literature data, 8.9% [35] - 68% [29] of patients with EC have relatives who suffer from epilepsy or have a history of seizures, and up to 30% [13] have relatives with the presence of rolandic spikes on the EEG in the absence of seizures. The frequency of detection of spikes in siblings of probands and children whose parents suffered from EC is 25-36% (in a healthy population - 1.4-5% [37]). However, only in a small part of them (12% in R. Bray's study and W.Wiser [13]) had seizures: the rest were clinically healthy. The nature of seizures in relatives of patients with rolandic epilepsy may be different. Typical seizures characteristic of RE are found in 13% of relatives, absence epilepsy - in 10%, generalized convulsive seizures - in 3%, focal epilepsy - in 1% [9].
A number of studies have examined the concordance of monozygotic twins for EC. The highest concordance was found for the presence of rolandic spikes on the EEG, but not for the development of RE itself. T. Kajitani et al. [30] studied 3 pairs of monozygotic twins aged 5-11 years, in which one of the siblings suffered from EC. In all cases, typical Rolandic epileptic activity was detected on the EEG, but none of the healthy siblings had any manifestations of RE.
In recent years, a hypothesis has been put forward about the multifactorial inheritance of rolandic epilepsy [29]. It is based on the fact that only a small proportion of children with Rolandic spikes on the EEG subsequently develop RE. It is assumed that rolandic adhesions and the development of EC are determined by two different but linked genes. In 1982, O.Eeg-Olofsson et al. [22] found that parents, siblings and other relatives of children with EC have a low incidence of the A1B8 haplotype of leukocyte histocompatibility antigens. Its frequency in the general population is extremely high [22]. It is possible that this haplotype plays the role of a protector or inhibitory factor that prevents the development of EC.
Interesting studies reveal the relationship between RE and febrile seizures. E. Frantzen et al. [24] reported that 20% of children with febrile seizures subsequently develop focal epileptic activity on the EEG, identical to rolandic spikes. None of these patients had subsequent epileptic seizures. Studies in monozygotic twins have also suggested a possible genetic linkage between EC and febrile seizures. M. Lennox-Buchthul [32] studied 24 pairs of monozygotic twins in which at least 1 of the pair had febrile seizures, and found that 20% of their siblings had rare nocturnal seizures. In 2 out of 24, a combination of nocturnal attacks with febrile convulsions was found. Unfortunately, the author did not identify these nocturnal attacks, however, based on the nature of their course and the age of onset, it can be assumed that these are cases of RE. In a study by T. Kajitani et al. [31] among 3 pairs of monozygotic twins, 3 had EC and 5 had febrile seizures. These studies may indicate a genetic linkage between EC and febrile seizures. This hypothesis is also confirmed by the clinical similarity of these two syndromes: high genetic determination, “hard” age-dependent onset, benign prognosis [1].
The role of exogenous, environmental factors in the development of Rolandic epilepsy remains unclear. H. Doose and W. Baier [18] suggested that genetic factors in EC determine a low threshold for convulsive readiness of the brain. At the same time, they regarded healthy children with Rolandic spikes on the EEG as subclinical carriers with a low seizure threshold. According to the authors, the influence of various exogenous factors in ontogenesis, as well as linkage with other genes, may contribute to the clinical manifestation of the disease in such patients and have some influence on its course. T. Kajitani et al. [30] described 3 siblings, each of whom had typical Rolandic spikes on the EEG. In one of the siblings, RE debuted at the age of 4 during a febrile illness, another had a single attack at the age of 10 while watching television, the third had passed the “critical period” for the debut and was healthy. This observation illustrates the polymorphism of the clinical manifestations of the disease, which may depend on various exogenous influences.
Factors that transform subclinical carriage into EC may be pathology of pregnancy and childbirth, head injuries, neuroinfections, etc. [5]. However, in more than half of children suffering from EC, there is no indication of the presence of these factors in the anamnesis[15].
Electroencephalography
A necessary study to objectify the diagnosis of EC is electroencephalography [14]. The EEG in the interictal period with EC reveals characteristic, typical “rolandic” or “rigidly” localized: the central and centritemporal regions. Rolandic complexes can be observed both unilaterally (usually contralateral to hemifacial seizures) - 60% of patients, and bilaterally - 40% [29]. Epileptic complexes are usually independent of each other, only in rare cases is their bilateral synchronous distribution with amplitude observed predominance on one side [26,34].
D. Gregory and R. Wong [28] studied the exact localization of rolandic adhesions using a computerized topographic mapping method. The authors found that the maximum “positivity” of the electric dipole in RE is located in the central-temporal region, and the maximum “negativity” is in the frontal region. It is assumed that specific EEG patterns in RE originate from a zone located in the lower parts of the Rolandic fissure, on the border with the Sylvian fissure. A typical feature of EEG changes in RE is the instability of patterns, their variability from one recording to another. Rolandic spikes can disappear and then appear again, changing their harmony and configuration even with two subsequent EEG recordings after a short period of time. The instability of EEG patterns in EC indicates, according to J. Aicardi [5], the absence of organic changes in the brain in EC. In this regard, the absence of rolandic spikes during a single electroencephalographic study cannot be considered a convincing argument for excluding the diagnosis of EC in the presence of a typical pattern of seizures [15]. An EEG study during sleep is extremely important to confirm the diagnosis of EC. About 30% of children suffering from EC have rolandic adhesions exclusively during sleep [29]. During drowsiness and at all stages of sleep, there is a tendency for the unilateral Rolandic complexes to transform into bilateral ones [34].
An interesting finding in RE is the detection on the EEG, along with typical Rolandic complexes, of other epileptic patterns. From 10 to 20% of children with EC have peak-wave complexes on the EEG in other areas of the cortex, mainly in the occipital region [41]. The morphology of the “occipital complexes” is close to the Rolandic ones and to those observed in benign focal epilepsy with occipital paroxysms [10]. According to J. Aicardi [5], the frequency of “occipital complexes” with Reo is fraternally proportional to the age of the child and is not uncommon in patients under 3 years of age. From 7% [34] to 20% [5] of patients with EC show typical generalized peak-wave activity on the EEG with a frequency of 3-4 Hz, often occurring during hyperventilation. In some patients, slow peak-wave complexes may appear. Most authors are of the unequivocal opinion that there is no correlation between the frequency and severity of peak-wave complexes on the EEG and the characteristics of the course of the disease.
The study of EEG during an attack seems quite difficult due to the low frequency of attacks in RE. B. Dalla-Bernardina et al. [15] described during a night attack the appearance on the EEG of low-amplitude fast activity in the central-temporal region, turning into the Rolandic complexes spreading to the entire hemisphere and subsequently covering the entire hemisphere.
Neuroradiological study
Information about neuroradiological studies in patients with EC is rare. H. Gastaut [25] was one of the first to study computed tomography data in 15 patients with EC and did not find pathological changes in any case. Subsequently, a neuroradiological study in patients with EC revealed some structural changes in the brain: widening of the Sylvian fissure, the presence of a cavity of the septum pellucida (2 of 13 cases) [39], ventriculomegaly, arachnoid cysts (3 of 17 cases) [38]. R. Santanelli et al. [43] presented a detailed clinical and neuroradiological description of 3 cases of EC associated with structural changes in the brain. One patient was diagnosed with a lipoma of the corpus callosum, another (cospondylothoracal dysplasia) had agenesis of the corpus callosum with ventriculomegaly, and a third had multiple cerebral, predominantly periventricular, calcifications (congenital toxoplasmosis). All these patients had a typical clinical picture of RE and rolandic spikes on the EEG.
Organic symptoms of brain damage discovered during neuroradiological examination in isolated patients with EC are difficult to interpret unambiguously. It is possible that these disorders do not have a direct cause-and-effect relationship with EC and may be caused by a combination of EC with other diseases - cerebral palsy, microcephaly, toxoplasmosis, ectomesodermal dysplasia. As in the general population, patients with EC may experience perinatal damage to the central nervous system and postnatal traumatic brain injury. The frequency of perinatal lesions of the central nervous system among patients with EC is 6% [16] - 13% [34], concussion - 4 - 5% [34], which does not exceed statistical data in the population of healthy children. A fundamentally important and not fully resolved question is whether these exogenous factors influence the occurrence and course of EC. According to J. Aicardi [5], disorders found in the brain of patients with EC cannot be the cause of epilepsy, but are random findings. On the other hand, one cannot ignore the fact that in a number of patients with EC, structural changes in the brain are localized in the lower parts of the Rolandic sulcus [43], and this does not completely exclude the possibility of the existence of a “symptomatic” form of epilepsy.
Treatment
In recent years, a number of antiepileptic drugs have been tested in the treatment of EC. Most authors recommend the use of drugs that primarily affect partial forms of epilepsy: carbamazepine (finlepsin, tegretol) and diphenin (phenytoin). When treating EC, it is necessary to avoid polytherapy, as well as the use of anticonvulsants in high doses. The benign course of RE and the absence of intellectual and mnestic disorders in patients do not provide grounds for recommending “aggressive” anticonvulsant polytherapy for the treatment of this form of epilepsy due to the possibility of developing severe side effects.
According to P. Lerman [34], as well as V. Dalla-Bermardina et al. [15], one of the drugs of choice for EC is phenytoin in low and medium doses. If attacks occur exclusively at night or during awakening, a single dose of phenytoin at night is recommended. In all cases observed by the authors, the use of moderate therapeutic doses of phenytoin led to complete clinical remission of attacks. According to P. Lerman and S. Kivity [33], the effectiveness of phenytoin in the treatment of EC exceeds that of carbamazepine and phenobarbital.
Carbamazepine is also highly effective in the treatment of EC [41] and differs from diphenine in the minimal severity of side effects of therapy.
Sulthiam (opolot) is effective and well tolerated by patients with EC [19]. The use of barbiturates (phenobarbital, benzonal) is limited by frequent and severe side effects on the intellectual-mnestic functions and behavior of children [5] and cannot be recommended for the treatment of EC.
In recent years, publications have appeared indicating the effectiveness of valproic acid drugs (Depakine, Conuvulex) for EC [6,15,29]. An important feature of them is their positive effect on the intellectual and mnestic functions of patients and good tolerability [12]. However, there are no follow-up studies of the effectiveness of valproate use in EC.
Most authors believe that the duration of use of anticonvulsants for EC should not exceed 2 years from the date of the last attack [35]. The average duration of drug treatment for EC is 3.2 years [33]. However, in case of early onset of attacks (2-3 years), treatment should be continued until 10 years of age, regardless of the duration of remission [4]. Treatment is recommended to be prescribed only after a recurrent attack. The persistence of typical epileptic activity on the EEG cannot be a reason to continue anticonvulsant therapy if the patient has a long-term remission.
Forecast
A feature of EC is the good prognosis of the disease [20]. According to P. Loisea et al. [35], after 13 years, RE attacks disappeared in 93.5% of 168 examined patients, and after 16 years - in 98.8%. P.Loisea et al. [35] studied in detail the influence of individual clinical symptoms of EC on the prognosis of the disease. As possible factors influencing the prognosis, the gender of the patients, the age of onset of epilepsy, the frequency and nature of attacks, their daily distribution, and features of the neurological status were analyzed. All 168 patients with EC were followed up for at least 4 years since the last attack. Factors influencing the prognosis of EC were identified: age of onset and frequency of attacks. It was found that only the age of onset of epilepsy statistically significantly correlates with the prognosis of EC. It has been established that the duration of the disease and resistance to therapy increase with early (before 4 years) and late (after 10 years) onset of epilepsy. All other factors do not significantly affect the prognosis of RE.
Long-term follow-up observation of patients revealed the possibility of relapses of attacks with EC after a long remission. The rate of relapse of attacks in adulthood with EC is low - from 1.8% [35] to 4% [34]. Relapse of attacks is more often observed in women during the period of hormonal changes, 3–10 years after the onset of remission, and is not associated with the use of anticonvulsants [35]. In most cases, generalized convulsive attacks appear, isolated or rare. An important feature is the absence of typical Rolandic spikes on the EEG; according to P. Loisea et al. [35], the EEG in these patients is usually within normal limits.
Considering the generalized nature of the attacks, their appearance long after the onset of remission, and the absence of rolandic spikes on the EEG, P. Lerman [34] suggested that these cases are not related to RE and represent the occurrence of de novo epilepsy. This concept is contradicted by the fact that the relapse rate in EC (2-4%) significantly exceeds the population frequency of epilepsy (0.8%). It is possible that the interaction of exogenous factors with a genetically determined decrease in the threshold of convulsive readiness plays a role in the occurrence of seizures in adulthood with EC. One of the “resolving” factors may be a hormonal imbalance in the body.
Another possible cause of recurrent attacks may be the existence of persistent structural changes in the brain localized in the lower parts of the Rolandic or Sylvian fissure. G. Ambrosetto et al. [6] described a recurrence of typical “rolandic” seizures in a patient aged 21 years. The patient suffered from RE since the age of 10: nocturnal simple partial and secondary generalized seizures. At the age of 13, she went into remission; at the age of 16, she stopped taking anticonvulsants. At the age of 21, for no apparent reason, the patient developed exactly the same partial and secondary generalized seizures at night with a frequency of 1-4 per month. Computed tomography revealed no pathology. EEG at the age of 21 and 30 years (in dynamics) shows a right-sided focus of typical rolandic activity. The uniqueness of this observation is that in the patient with EC, attacks reappeared 8 years after the onset of remission and were of a typical nature for EC, being, however, resistant to anticonvulsants. According to the authors, in this case there is a high probability that the patient has structural changes in the rolandic region, which can be detected with a more thorough neuroradiological examination (nuclear magnetic resonance, positron emission tomography).
Further studies will show the relationship between genetic and exogenous factors in the determination of EC and the possibility of the existence of “symptomatic” forms of EC caused by structural changes localized in the rolandic region. It is also necessary to conduct molecular genetic studies to search for genes responsible for the development of EC. This will make it possible to determine the relationship between RE and other forms of idiopathic epilepsy, such as benign occipital Gastaut epilepsy, childhood absence epilepsy, etc.
LITERATURE
I. Badalyan L.O., Temin P.A., Mukhin K.Yu. Febrile seizures. Guidelines. M. 1987; 24.
2. Karlov V.A. Epilepsy. M. 1990; 219 - 223.
3. Sarajishvili P.M., Geladze T.Sh. Ibid. 1977, 148 - 149.
4. Aicardi J.J., Chevrie J.-J. Dev Med Child Neurol 1982, 24: 281 – 292.
5. Aicardi J. Epilepsy in children 1986; 4.
6. Ambrosetto G, Tenuper P, Baruzzi A. J Neural Neurosurg Psychiat 1985; 48:90.
7. Bancaund J, Collomb D, Dell MB Rev Neurol 1958; 99: 206 - 209.
8. Beaumanoir A., Ballis T., Varfis G., Ansai K. Epilepsia l974; 15: 301 - 315.
9. Beaussart M., Favou R. Ibid 1978; 19: 337 - 342.
10. Beydoun A»index.html» Garofalo EA»index.html» Drury I. Ibid 1992; 33: 1091 – 1096.
11. Blom S., Heijbel J. Ibid 1982: 23: 629 - 631.
12. Bourgeois BFD Ibid 1994. 35: 2: 18 - 23.
13. Bray PP, Wiser WC Pediatrics 1965; 36: 207 - 211.
14. Commissionon Classification and Terminology of the ILAE. Epilepsy 1989; 30: 389 -399.
15. Dalla-Bermardina V., Sgro V., Fontana E. Epileptic syndromes in infancy, childhood and adolescence. Paris 1992; 173 - 186.
16. DeonnaTh., ZieglevA-L., Despland P.-A. Neuropediatrics 1986; 17: 144 - 151.
17. Deonna Th., Zieglev A.-L., Despland P.-A. Guyvan Melle. Epilepsy 1986; 27: 241-247.
18. Doose N, Baier WK Europ J Pediatr 1989; 149: 152 – 158.
19. Doose H. Epileptic syndromes in enfancy, childhood and adolescence. Paris 1992; 103-114.
20. Dreifuss FE Epilepsia 1994; 35: 2: 30 – 34.
21. Dulac O., Cusmai R., Olivera K.de. Ibid 1989; 30: 798 – 801.
22. Eeg-Olofsson O., Safwenbery J., Wigrtz A. Ibid 1982; 23: 27 – 34.
23. Fejerman N., Di Blasi AM Ibid 1987; 28: 351 - 355.
24. Frantzen E., Lennox-Buchthal M., Nygaakt A. Electroencephalogr Clin Neurophysiol1968; 24: 197 - 212.
25. Gastaut H. Rev Neurol 1952; 87: 488 – 490.
26. Gastaut H, Gastaut JL Epilepsia 1976; 17: 325 - 326.
27. Gibbs EL, Gibbs FA Ibid 1960; 1: 448 – 453.
28. Gregory DL, Wong PK Ibid 1984, 25: 705 - 711.
29. Heijbel J. Pediatric Epilepsy. Helsinki 1989.
30. Kajitani T, Nakaltmra M, Llcaka K, Kobuchi S. Advances in Epileptology 1980; 4:171 - 175.
31. Kajitani T, Kimura T, Sumita M, Kaneco M. Brain Dev 1992; 14: 230 - 234.
32. Lennox-Buchthul M. Epilepsia 1971, 12: 197 - 212. 33. Lerman P., Kivity S. ArchNeural 1975; 32: 261 - 264.
34. Lerman P. Epileptic syndromes in infancy, childhood and adolescence. Paris 1992;173 – 186.
35. Loisea P., Duche V., Cordova S. et al. Epilepsy 1988; 29: 229 - 235.
36. Lombroso CT Arch Neurol 1967; 17: 52 - 59.
37. Luders N.O., Lesser RP, Dinner DS, Morris HH Epilepsy - electroclinical syndromes. Berlin 1987; 303 - 346.
38. Mambelli M., Moscano F., Maroni P. et al. Bull. Liga Int. Epilepsy 1985; 51/52: 79-80.
39. Morikawa T, Osawa T, Ishihara O, Seino M. Brain Dev 1979; 1: 303 – 346.
40. Nayrac P, Beaussart M. Rev Neurol 1958; 99: 201 - 206.
41. Panayiotopoulos S.R. J Neurol Neurosurg Psychiat 1993; 56: 2 - 5.
42. Roulet E, Deonna Th, Despland PA Epilepsia 1989; 30.564 - 568.
43. Santanelli P., Bureau M., Magaudda A. et al. Ibid 1989; 30: 182 - 188.
Treatment of benign rolandic epilepsy
In neurology, the issue of treatment of benign rolandic epilepsy remains controversial. This is due to the fact that in children with age, all symptoms of the disease disappear without additional medical intervention. Therefore, some doctors defend the opinion that it is inappropriate to prescribe antiepileptic therapy. The opposite opinion is based on the complexity of differential diagnosis of the disease. It may happen that the diagnosis is made incorrectly or the pathology progresses to the pseudolennox stage. To prevent possible complications, doctors may still prescribe antiepileptic therapy to the patient.
When treating children with benign rolandic epilepsy, the principle of monotherapy is used - they are prescribed only one drug. Typically, treatment of children begins with the administration of valproic acid (Depakine, Convulsofin, Convulex). If treatment is ineffective or the child is intolerant to this drug, it is replaced with Topamax or levetiracetam. Children over seven years of age may be prescribed carbamazepine.
Parents often do not immediately notice single or rare epileptic seizures in children, since in most cases they occur at night. However, immediately after detecting epileptic seizures, the child must be shown to a neurologist. Diagnosis and treatment of pathology are selected for each case individually. The most careful monitoring is required for patients under three years of age, who may experience an increase in seizures due to the immaturity of nerve cells.
Parents of a sick child who has had an attack also need to know what they can do on their own before the ambulance arrives. If the baby has a complex seizure, which is accompanied by serious motor disturbances (tonic convulsions or muscle twitching of the face, tongue, larynx and pharynx), he needs to be slightly raised and calmed. It is extremely important to do this if the attack occurs while drinking, eating or sleeping. To prevent the tongue from sticking, you need to put the child's head on one side, and then put a handkerchief or some soft object in his mouth. If a child has a prolonged seizure, it is necessary to urgently call an ambulance.
Epilepsy in children
Epilepsy is one of the most common neurological diseases in the world. People of all ages are susceptible to it, but in children various epileptic manifestations are three times more common. The first epileptic seizure occurs in 78% of children.
There are about 60 types of epilepsy. A child diagnosed with epilepsy may be no different from his peers, not have any seizures, play and develop according to his age. But it’s bad to study at school because he “stares” at one point for 15 seconds and misses information in class (absences). Or an infant with West syndrome with seizures that are difficult to distinguish from the normal motor activity of a newborn.
Such convulsions can be repeated up to 100 times a day, and each time the electrical discharge will destroy the baby’s developing brain. Although outwardly these seizures do not seem to be anything serious, the child simply spreads his arms to the sides. Such a child does not develop and cannot even hold his head up. This leads to lifelong disability of the baby with subsequent death. Therefore, it is incorrect to talk about epilepsy as one disease, and approaches to its treatment will be different.
Recommendations for epileptic seizures
There are a number of recommendations from doctors, adherence to which will help prevent an epileptic attack. First of all, you need to strictly adhere to your sleep and wakefulness schedule. The child must get a good night's sleep, as sleep problems can trigger a severe attack. In most cases, epileptic seizures occur in a child during sleep. At the same time, it is not always possible to notice an attack (usually this becomes clear only if the patient bites his tongue during an attack). Therefore, if the baby sleeps in a separate room, it is advisable to use a baby monitor - a device that allows you to hear sounds in the next room.
Patients with Rolandic epilepsy often experience insomnia at night and drowsiness during the day. As for drowsiness, it occurs mainly due to the use of medications. Insomnia can be caused by poor sleep hygiene: the patient should not sleep in a stuffy room, with the TV on, and should not drink or overeat before bed. The child should go to bed at a set time. If all these recommendations do not help and the attacks recur regularly, you should consult a neurologist.
Diagnosis of epilepsy
Epilepsy is diagnosed using an EEG (Electroencephalogram), the picture shows jumps in Theta waves and dysrhythmia. An experienced neurologist-epileptologist should decipher the EEG. The gold standard in epileptology is nightly 12-hour video monitoring of a child’s sleep.
Only in this case is it possible to determine with 90% certainty the nature of the attack, the area of distribution of the discharge, and its presence in principle. During a daily routine EEG, in half an hour the presence of epilepsy can be detected only in 30% of cases, and even less if the diagnosis is carried out on an infant.
If epileptic activity is detected in children, the doctor prescribes an MRI of the brain. Magnetic resonance imaging will help determine whether the child has organic brain lesions, tumors, cysts and other anomalies that generate an electrical discharge.
It is also necessary to conduct a blood test for deficiencies of microelements and vitamins (especially magnesium, zinc, omega-3). The relationship between vitamin deficiency and epileptic seizures in children has been clinically proven.
Surgical treatment of epilepsy
It is used in cases of focal epilepsy with organic brain lesions or neoplasms. Treatment for epilepsy with surgery is used to either remove a tumor in the brain or remove the part of the brain where the electrical discharge occurs.
Surgical intervention is a last resort, but success is achieved in 85% of cases. Only 20% of patients suffering from epileptic seizures are indicated for surgical intervention. Treatment of epilepsy is carried out using the following methods:
- Subpial incisions
- Lobectomy
- Neurostimulator implantation
- Functional hemispherotomy
- Removal of pathological formation (2)
Drug treatment of epilepsy
Traditionally, the range of drugs used to treat epilepsy is aimed at:
- pain relief for seizures
- reducing the duration of an epileptic seizure
- reduction in the frequency of attacks
- stopping the emergence of new cases of epilepsy
With epilepsy, AEDs in many cases have to be taken for life. One third (approximately 30%) of people with epilepsy continue to have seizures despite the use of various combinations of major antiepileptic drugs.
For the treatment of epilepsy use:
- Psychotropics - act on the central nervous system and change consciousness and mental activity, higher brain functions
- Neurotropes - activate or inhibit the transmission of impulses between neurons
- Racetams are a relatively new subclass of nootropic drugs that are being actively studied and considered promising in the treatment of epilepsy.
The selection of PEP can take a long time. To treat epilepsy, in some cases it is necessary to change the drug every month.
Active ingredients in anticonvulsants:
- barbiturates
- phenytoin
- carbamazepine
- lamotrigine
- levetiracetam
- ethosuximide
- valproic acid
Unfortunately, each drug has a large number of side effects; such treatment has a particularly negative effect on children suffering from epilepsy.
About side effects from drug treatment of epilepsy in children:
- hyperactivity
- irritability
- sleep structure disorder
- drug addiction
- ataxia
- dyskinesia
- liver damage
- aplastic anemia
- changes in connective tissue (leads to roughening of facial features, stiffness of joints)
- anorexia, weight loss
- vomit
- acne
- gingival hyperplasia
- osteoporosis
- general intoxication
- cerebellar atrophy
- severe developmental delay
- systemic lupus erythematosus
- depression
- memory loss
- psychoses
- irreversible concentric narrowing of visual fields, etc. (1)
Ketogenic diet for the treatment of epilepsy in children
At our center, we advocate the use of a ketogenic diet in patients with epilepsy. The course format (2-3 weeks) does not allow us to put the child on a diet. However, we strongly advise parents to consult a doctor upon arrival home and begin the process of treating epilepsy with diet. In 10-15% of cases, children with pharmacological resistance achieve remission within 2 years.
The ketogenic diet is not just a diet for epilepsy, it is a treatment. Any slight deviation from the diet, even the use of toothpaste that contains sugar, affects its effectiveness. The diet is prescribed by a doctor, accompanied throughout the entire treatment of epilepsy (usually 2 years) by a nutritionist, and begins in the hospital with a 24-hour fast.
The diet was developed in 1920 to alleviate epileptic seizures in children. It almost excludes carbohydrates, the basis of the diet is fats and a moderate amount of protein foods. The ratio of fat to protein + carbohydrates is 4 to 1.
The ketogenic diet in the treatment of epilepsy works in 50% of all cases, 34% of children have good, lasting results after its use. Almost all children (more than 90%) show a marked improvement in their condition within 6 to 24 months of using the diet. (3, 4)