History of the study
The corpus callosum has long remained a mystery of human anatomy. Scientists could not determine exactly what function this part of the brain has. By the way, in 1981, the scientist who discovered the corpus callosum received the Nobel Prize for this. His name was Roger Sperry.
The first operations on the corpus callosum were aimed at treating epilepsy. Thus, by disrupting the connection between the hemispheres, doctors actually cured many patients from epileptic seizures. But over time, scientists drew attention to the occurrence of specific side effects in such patients - behavioral reactions and abilities changed. Thus, as a result of experiments, it was found that after an operation affecting the corpus callosum, a person could write exclusively with his right hand, and draw only with his left. So the corpus callosum, the functions of which were still unknown to scientists, was no longer dissected in surgery to treat epilepsy.
A few years later, scientists discovered a connection between the focus of the corpus callosum and the development of multiple sclerosis.
Structure
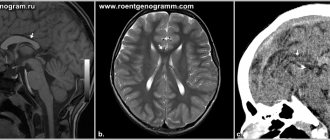
MRI of the corpus callosum and its named parts Corpus callosum
The corpus callosum forms the bottom of a longitudinal fissure that separates the two hemispheres of the brain. Part of the corpus callosum forms the roof of the lateral ventricles.[5]
The corpus callosum consists of four main parts; separate nerve pathways connecting different parts of the hemispheres. These stands
, then
Genu
, then
trunk
, or
body
, and
splendor
.[4] The narrowed part between the trunk and the asterisk is known as
the isthmus
.
The trunk and lamina fibers, known together as the tapetum,
form the roof of each lateral ventricle.[6]
The anterior part of the corpus callosum towards the frontal lobes is called the genu ("knee"). The knee bends down and back in front of the septum pellucida, greatly decreasing in thickness. The lower, much thinner part is the rostrum and is connected inferiorly to the lamina terminalis, which extends from the interventricular foramen to the notch at the base of the optic stalk. The rostrum is named because of its resemblance to a bird's beak.
The terminal part of the corpus callosum towards the cerebellum is called the asterisk. This is the thickest part, it overlaps the tela choroidea of the third ventricle and midbrain, and ends in a thick convex free border. Splenium translates to bandage
to Greek.
The trunk of the corpus callosum lies between the asterisk and the knee.
In the callosal groove
separates the corpus callosum from the cingulate gyrus.
communications
On either side of the corpus callosum, fibers diverge into the white matter and move to different parts of the cerebral cortex; those that curve forward from the knee into the frontal lobes constitute the minor forceps
(also
anterior forceps
) and those that bend backward from the asterisk into the occipital lobes, then
the forceps are large
(also
posterior forceps
).[4] Between these two parts is the main part of the fibers that make up the tapetum and pass laterally on both sides into the temporal lobe, and cover the central part of the lateral ventricle. The tapetum and anterior commissure share the function of connecting the left and right temporal lobes.
The anterior cerebral arteries contact the inferior surface of the rostrum, they curve over the front of the knee and run along the trunk to supply the anterior four-fifths of the corpus callosum.[7]
Neuron fibers
The size, degree of myelination, and fiber density of subregions are related to the functions of the brain regions they connect.[8] Myelination is the process of covering neurons with myelin, which helps transmit information between neurons. This process is believed to continue until the age of thirty, with peak growth occurring in the first decade of life.[9] Thinner, lightly myelinated fibers conduct more slowly and connect the association and prefrontal areas. Thicker, faster-conducting fibers connect the visual and motor areas.[10]
The tractogram The figure shows the nerve pathways from the six segments of the corpus callosum, providing the connection of the cortical areas between the cerebral hemispheres. The genu are shown in coral, the premotor in green, the sensorimotor in purple, the parietal in pink, the temporal in yellow, and the stellate in blue.[11]
Thinner axons in the genu connect the prefrontal cortex between the two halves of the brain; these fibers arise from a fork-shaped bundle of tapetum fibers, the small forceps. Thicker axons in the trunk of the corpus callosum connect areas of the motor cortex, with proportionately more of the corpus callosum devoted to additional motor areas, including Broca's area. The splenium, which communicates somatosensory information between the two halves of the parietal lobe and the visual cortex of the occipital lobe, is the fibers of the forceps major.[12][13]
In a study of children aged five to eighteen years, a positive correlation was found between age and callosal thickness.[3]
Differences between the sexes
The corpus callosum and its relationship to sex has been the subject of debate in scientific and secular communities for over a century. Initial studies in the early 20th century claimed that torso sizes differed between men and women. This research, in turn, was questioned and eventually gave way to more advanced imaging techniques that seemed to refute the earlier correlations. However, advanced analytical techniques from computational neuroanatomy developed in the 1990s showed that sex differences were clear but limited to certain parts of the corpus callosum and that they were correlated with cognitive performance on certain tests.[14] An MRI study has shown that the cross-sectional area of the midsagittal corpus callosum, after accounting for brain size, is on average proportionately larger in women.[15]
Using diffusion tensor sequences on MRI machines, the rate at which molecules diffuse into and out of a specific area of tissue, anisotropy can be measured and used as an indirect measure of the strength of an anatomical connection. These sequences revealed consistent sex differences in the shape and microstructure of the human corpus callosum. [ which?
][16][17][18]
Shape and size analysis have also been used to examine specific 3D mathematical relationships with MRI and have found consistent and statistically significant differences between genders.[19][20] Specific algorithms identified significant differences between the two genders in more than 70% of cases in one review.[21]
Localization
Spatially, this part of the brain is located under the hemispheres along the midline. From anterior to posterior, the corpus callosum can be divided into several different zones: genu, midsection, body, posterior end, and splenium. The knee, curving downwards, forms the beak, as well as the rostral plate. On top, the corpus callosum is covered with a thin layer of gray matter.
Another structure of this part of the brain is radiance. Strands of fan-shaped neurons extend to the frontal, parietal, temporal and occipital lobes of the cerebral hemispheres.
What it is
Like chemical elements that are connected by different types of connections, the left and right hemispheres of the telencephalon are connected to each other by the corpus callosum. This structure acts as a connecting bridge between two parts of the brain.
The corpus callosum is a structure consisting of clusters of nerve fibers - axons (up to 300 million), and is located under the cerebral cortex. This formation is unique to mammals. The body consists of three parts: the posterior section is the roller, the anterior section is the knee, which later turns into the key; The trunk is located between the roller and the knee.
Agenesis of the corpus callosum
With agenesis, the corpus callosum of the brain is completely or partially absent. This brain abnormality can be caused by a number of different factors, including chromosomal mutations, genetic inheritance, intrauterine infections, as well as other reasons that have not yet been fully studied by scientists. Individuals with agenesis of the corpus callosum may experience cognitive and communication impairments. They also have difficulty understanding spoken language and social cues.
But, given the functions that the corpus callosum of the brain performs, how can people who do not have it from birth even live? How do they interact between the right and left hemispheres of the brain? Scientists have found that at rest, the brain activity of a healthy person is practically no different from that of a person diagnosed with agenesis of the corpus callosum. This fact indicates that the brain is rebuilt under these conditions, and the functions of the absent corpus callosum are performed by other healthy areas. How exactly and through what structures this process is carried out, scientists have not yet figured out.
Text of the book “Neuropsychological analysis of the pathology of the corpus callosum”
Maria Kovyazina Neuropsychological analysis of the pathology of the corpus callosum
© Genesis Publishing House, 2012
* * *
Dedicated to my dear and beloved parents
Introduction
Since Roger Sperry's Nobel Prize
, 1964), awarded for studies of patients with split-brain syndrome, the problem of interhemispheric asymmetry and interhemispheric interaction continues to remain one of the central problems of the entire complex of modern neurosciences. This is an interdisciplinary problem, which, along with neuropsychology, is studied by neuroanatomy, neurobiology, neurophysiology, neurology, psychiatry, evolutionary biology, linguistics and other disciplines.
The number of experimental works on this problem continues to grow. Based on the results obtained, various local models for specific mental processes are proposed. Clinical and empirical data interpreted using these models are often inconsistent ( Kotik
, 1992). It should be noted that all local models still leave open such important questions as functional unilaterality and bilaterality of the hemispheres; the leading role of the hemispheres in the implementation of certain functions; material specificity of the hemispheres; ways of processing information by the hemispheres.
A. R. Luria supported the idea of cooperative interaction between the hemispheres. That is why today it is important to develop the principles of interhemispheric interaction, and not to contrast the hemispheres according to their functions, especially since the diverse hemispheric dichotomies are descriptive in nature and do not explain anything ( Kotik
, 1992).
The main theoretical concept of the Russian neuropsychological school is the theory of systemic dynamic brain localization of higher mental functions ( Vygotsky
, 1960;
Luria
, 1969). In modern neuropsychology, interhemispheric relations are understood as a dynamic process, the basic mechanism of implementation and compensatory reserve of any mental function (elementary and higher), to which each hemisphere makes its own specific contribution. The structures of the hemispheres play their role in the implementation of any mental function. It is in this differentiated participation and specific contribution of various brain structures of both hemispheres that the principles of systematic and dynamic cerebral organization of the psyche are realized. It must be emphasized that the principle of the dynamism of interhemispheric relations can be revealed only through the analysis of a specific task that a person is solving at the moment, and not through the characteristics of dominance - subdominance of the hemisphere, stimulus material, processing method, or even the level of occurrence of a particular mental process. Different parts of the brain can dynamically unite (interact) to solve a specific task, and their ratio and relationships (including interhemispheric ones) can change if necessary.
Any mental function is based on the structures of both hemispheres and is realized when they work together. As A. R. Luria writes in the preface
to the book by E. G. Simernitskaya “Hemisphere Dominance”: “...there is a close interaction between both hemispheres, and the role of each can change depending on the task to which mental activity is aimed, and on the structure of its organization” (
Simernitskaya
, 1978, p. 6).
By changing the psychological structure of activity, “it is possible to achieve not only a smoothing of interhemispheric asymmetry, but also a change in its sign to the opposite with the transition of the leading role in the implementation of the same activity from the left to the right and from the right to the left hemisphere of the brain” ( Simernitskaya
, 1978, p. . 10).
Recognition of the dynamic nature of the functional interaction of the cerebral hemispheres makes it necessary to study specific neuropsychological factors to clarify the lateral functional organization of the cerebral hemispheres and to correlate their “own functions” with specific areas and structures of the brain ( Luria
, 1969;
Chomskaya
, 1986), and not higher mental functions (HMF) of a person and tasks solved by a person.
The principle of the dynamism of the brain organization of the psyche is based on the property of multifunctionality of various brain structures. “...According to our ideas, this or that structure is involved primarily in ensuring the basic, genetically inherent functions. In accordance with this, it is assumed that different formations and systems of the brain are characterized to varying degrees by the interaction of two forms of structural organization and activity - invariant, genetically determined and mobile, probabilistic. The implementation of functions is also based on the differences and complex hierarchy of relationships that exist between different (projective, associative, integrative-trigger, limbic-reticular) systems of the brain” ( Adrianov
, 1986, pp. 10–11). Subcortical, intrahemispheric and interhemispheric interactions require careful correlation of brain structures with mental manifestations.
A lot of experimental and clinical data have been collected on models of local brain lesions on the functional specificity of the left and right hemispheres in relation to various mental functions. The features of hemispheric neuropsychological syndromes are clarified, and therefore the concept of “hemispheric factors” is filled with content, under which today the prevailing ideas regarding the strategies of the left and right hemispheres of the brain can be interpreted ( Chomskaya
, 1986).
Coordination of the diverse effects contributed by the right and left hemispheres is possible only if there is a special mechanism or mechanisms of interhemispheric interaction. It is the presence of functional connections between the hemispheres that provides the advantages of the brain as a paired organ. At the same time, it becomes inappropriate to explain the observed neuropsychological symptoms in unilateral brain lesions only by a deficit in the functions of the damaged hemisphere without taking into account changes in the states of interhemispheric interaction and the intact cerebral hemisphere ( Traugott
, 1986).
There are not many studies aimed at studying interhemispheric interaction. The nature of the dynamism of interhemispheric interaction and the change in the role of each hemisphere in this interaction when performing different types of mental activity remain poorly understood. “...It is unclear how specifically the disturbance of interhemispheric interaction manifests itself, what functions it concerns, what is its dependence on the lateralization and localization of the lesion, what are the ways to study it” ( Traugott
, 1986, p. 14). The role of the brain substrate that ensures this interaction is also unclear, and it is also unclear how it is disrupted in pathology of the commissural system, primarily the corpus callosum (CC). The functional connection of the commissural system (and primarily the MT) with the cerebral hemispheres is not clear. What factors (besides the age of the subject) determine this dynamic relationship?
Disclosure of the neuropsychological dynamic mechanisms of the functioning of the commissural system of the brain, its contribution to the implementation of any mental function is an important task of modern neuropsychology. Neuropsychologists have long talked about the existence of a “factor of interhemispheric interaction” ( Chomskaya
, 1987), but this concept is not yet sufficiently filled with specific content. The question remains open about the qualitative specificity of neuropsychological syndromes in various MT pathologies. Will they be fundamentally different from hemispheric syndromes? Or will they be partly similar to hemispheric syndromes and at the same time will include a number of specific symptoms, or will the combination of these symptoms itself be specific? In other words, I would like to understand whether the neuropsychological factors of interhemispheric interaction are “hemispheric” factors or constitute an independent group of factors? Or maybe both?
In solving the problem of interhemispheric interaction as a problem of Luriev’s neuropsychology, it is necessary to remember the concept of three functional blocks of the brain ( Luria
, 1973). According to this concept, the integrative work of the three brain blocks underlies all mental activity and human behavior. This means that we can consider interhemispheric interaction as a process of dynamic interaction of three blocks with their constituent structures of the right and left hemispheres of the brain. And this, in turn, suggests that neuropsychological syndromes of impaired interhemispheric interaction are likely to include symptoms coming from all three functional blocks of the brain.
The problem of interhemispheric interaction remains poorly understood, as it consists of a number of difficult-to-solve particular problems. Interhemispheric interaction can be disrupted with pathology of one of the cerebral hemispheres and with pathology of the commissures connecting both hemispheres. That is, the anatomical substrate of interhemispheric interaction is the numerous cerebral commissures that form the commissural system. R. Sperry identifies MT, hippocampal commissure, frenulum commissure, posterior commissure, quadrigeminal commissure, cerebellum, mass intermedius, optic chiasm, anterior commissure ( Sperry
, 1964). In most anatomy textbooks, experts distinguish between the MT, the anterior commissure of the brain, the posterior commissure of the fornix, and the mass intermedius. The given examples of lists of commissures clearly demonstrate that the question is controversial, which anatomical formations are considered commissures? In particular, is the cerebellum or optic chiasm a commissure of the brain? To which functional blocks of the brain (according to the concept of A.R. Luria) do the commissures and, first of all, the MT belong?
Another particular problem remains “pure” clinical models, which make it possible to study precisely the processes of interhemispheric interaction. And here, along with models of commissurotomy and callosotomy, clinical cases of various pathologies of brain commissures, and primarily MT, provide a unique opportunity. Today, attempts are being made to describe a general set of behavioral symptoms in MT pathology, since the clinical picture of this pathology is very diverse and nonspecific. Unfortunately, here too one of the controversial issues of neuropsychology remains open - the question of the nature of the neuropsychological symptom and syndrome and its meaning. The qualitative specificity of the neuropsychological syndrome in MT pathology can be determined not only by the symptoms of impaired HMF, but also by compensatory symptoms. Neuropsychological qualification of these symptoms will make it possible to understand the hierarchical connections within the commissural system itself and the capabilities of each hemisphere in overcoming the deficit of interhemispheric relations. Are the identified symptoms a consequence of a deficiency in the functioning of the MT or are they responses of compensatory processes or functioning intact hemispheres? As N.K. Korsakova recalls: “Alexander Romanovich did not like to discuss this eternal question and the subject of dispute between different neuropsychological schools” (from a private conversation).
Possible mechanisms for compensating interhemispheric deficits in pathologies (especially congenital) MT are widely discussed in foreign literature ( Barr
, 2003).
Firstly, this is the bilateral representation of functions. This assumption is contradicted by some studies, which found that patients with congenital pathology of MT are characterized by normal functional asymmetry of speech and a normal distribution of handedness ( Sauerwein
et al., 1994).
Secondly, an increase in the volume of ipsilateral motor and sensory pathways. Milner argues against this hypothesis, showing that if there really was an increase in the volume of ipsilateral fibers, then information would reach both hemispheres simultaneously, and therefore, the reaction time to ipsi- and contralateral stimuli would be the same. Research shows, however, that this is not the case, and even in people with MT agenesis (AMT), reaction times to ipsilateral stimuli are slower than reaction times to contralateral stimuli compared to normal ( Milner
, 1994).
Thirdly, compensation due to extracallosal commissures of the brain. The most important role here is played by the anterior commissure, which is most often preserved in AMT ( Barr, Corballis
, 2002).
It is worth noting that compensatory mechanisms cannot completely replace MT (for example, with its agenesis or hypoplasia) ( Gott, Saul
, 1978). All the mentioned foreign studies once again confirm that in interhemispheric relations it is not so much the anatomical, but rather the functional dynamic connections between various anatomical structures of the brain that are important. In this context, these are functional connections of the cerebral hemispheres with the largest commissure - MT.
A big particular problem is the method that allows us to identify and evaluate symptoms of disturbances in interhemispheric interaction and study its mechanisms. As before, the most adequate and informative remains neuropsychological research, supplemented by experimental techniques (for example, dichotic listening and others). Thanks to this method, it is possible to analyze the contribution that the hemispheres and commissures of the brain make to interhemispheric relationships.
Understanding that the question of the joint work of the cerebral hemispheres in the implementation of human mental activity is the most controversial in neuropsychology, the author is aware that the proposed monograph does not fully answer it. The work makes an attempt to approach the problem of interhemispheric interaction from the perspective of its disturbances in various MT pathologies and offers material for discussing this complex and important problem.
The author expresses deep gratitude to N. K. Korsakova, Associate Professor of the Department of Neuro- and Pathopsychology of the Faculty of Psychology of Moscow State University and the Director of the Institute of Cognitive Research, Corresponding Member of the Russian Academy of Sciences, Doctor of Psychological Sciences, Professor B. M. Velichkovsky for their support and assistance in writing this work. The author is grateful to the senior research fellows of the Research Institute of Psychiatry of Roszdrav, candidates of medical sciences A. A. Zemlyanaya and L. V. Sokolova, leading researcher of the Research Institute of Psychiatry of Roszdrav, Doctor of Medical Sciences E. V. Zheleznova and leading researcher of the Scientific Center of Neurology of the Russian Academy of Medical Sciences, Doctor of Medical Sciences Sciences L.A. Kalashnikova, researcher of the Scientific Center of Neurology of the Russian Academy of Medical Sciences, Candidate of Medical Sciences L.A. Dobrynina, since most of the subjects (a total of 60 people with various MT pathologies were examined) were recruited at these clinical bases. The author is sincerely grateful to the entire Department of Neuro- and Pathopsychology of the Faculty of Psychology of Moscow State University named after M.V. Lomonosov, under the leadership of Doctor of Psychological Sciences, Professor A. Sh. Tkhostov for their attention and interest in this study.
Chapter 1. Formation of the corpus callosum and interhemispheric interaction in ontogenesis
Deep in the longitudinal fissure of the brain, both hemispheres are connected to each other by a thick horizontal plate consisting of nerve fibers running transversely from one hemisphere to the other. The plate is called the corpus callosum (CC)
or
corpus callosum (CC)
.
According to some data, the area of MT fibers is 663–664 mm2 ( Jaenke
et al., 1997).
This is the largest commissure of the brain, 7–9 cm long. The MT is divided into anterior, middle and posterior sections. The anterior section forms the knee of the MT (genu corpus callosi). It, curving downwards, sharpens and forms a keel or beak (rostrum), which passes into a thin plate of the lamina rostralis, which in turn continues into the lamina terminalis, which is located in front and below the anterior commissure of the brain (commissural anterior). The middle section, the MT trunk (truncus corpus callosi), forms a convexity in the longitudinal direction and is the longest part of the large commissure of the brain. The posterior section forms a thickening, the MT cushion (splenium corpis callosi). In the white matter of the hemispheres, the MT fibers diverge fan-shaped, forming the MT radiation (radiatio corpus callosi), which in front passes into the frontal (small) forceps, and behind into the occipital (large) forceps. On the upper surface of the MT there is gray matter (gray vesture), which forms four small longitudinal thickenings: two medial longitudinal stripes, which anteriorly pass into the beak area and the periterminal gyrus, as well as two lateral longitudinal stripes, which connect to the dentate gyrus of the hippocampus. The narrowed part of the cingulate gyrus of the cerebral cortex behind the MT splenium, which passes into the parahippocampal gyrus, is called the isthmus (isthmus) ( Bolycheva
, 2004).
Rice. 1 a
.
Radiation of the corpus callosum ( Bolycheva
, 2004): 1. anterior commissure; 2. corpus callosum; 3. radiance of the corpus callosum; 4. forehead forceps; 5. nuchal forceps
Rice. 1 b
.
Parts of the corpus callosum ( Bolycheva
, 2004): 1. splenium; 2. trunk; 3. knee; 4. beak; 5. gray vestments; 6. medial longitudinal stripe; 7. lateral longitudinal stripe
Commissural fibers running in the beak and partially in the knee of the MT connect similar areas of the cortex of the left and right frontal lobes. The knee of the MT contains fibers connecting similar areas of the cortex of the central gyri, parietal and temporal lobes of both hemispheres. The MT roll consists of commissural fibers connecting the cortex of the occipital and posterior parietal parts of the left and right hemispheres.
The MT begins to form at 10–11 weeks of gestation and first develops rostrally in the shape of a knee. Other parts of the MT, the beak and the ridge, are formed after the trunk appears. At the 16th week of the embryonic period, the shape of the MT becomes recognizable, like that of an adult. In its early development, the knee grows faster than the roll, which shows rapid growth only after birth. Thus, similar development of the splenium and posterior region of the MT may explain why they are susceptible to damage in the third trimester of perinatal pregnancy ( Nosarti
et al., 2004).
MT is formed in the direction from the frontal parts of the brain to the occipital and consists of myelinated fibers, a significant part of which are the axons of pyramidal neurons of layers 3–5 of the cortex ( Innocenti
, 1986).
In a study of rhesus monkeys, it was found that the number of MT axons increases before birth but decreases thereafter ( Lamantia and Rakic
, 1990). It is likely that the increase in MT in childhood is most likely due to myelination rather than an increase in the number of axons. Interhemispheric transmission time in humans for the shortest callosal axons is 100–200 ms; for axons forming the longest paths, almost 1/3 of a second.
A significant body of evidence reviewed by Innocenti (1986, 1994) shows that MT fibers occupy their positions as a result of anatomical competition for synapses, which in turn are controlled by the external environment and the internal environment of the body. Synapses in the right hemisphere are associated with the expansion and complexity of the internal and external perceptual fields of a person, and in the left hemisphere - primarily with the processes of speech development ( Thatsher
et al., 1987;
Semenovich
, 2001). The hemispheres develop relatively independently, constantly competing both anatomically and functionally. And hemispheric specialization arises from long-term integration of the multisynaptic process, which is achieved in the human brain through interhemispheric connections.
Morphological and electrophysiological studies described in a collective monograph ( Mosidze
et al., 1977), and modern work shows that MT fibers connect not only symmetrical areas of the cortex (homotopic connections), but there is a large array of heterotopic connections in MT (
Clarke S.
, 2003). Callosal fibers originating in the superficial layers of the cortex of one hemisphere end in the superficial layers of the opposite hemisphere, and fibers originating in the deep layers end in the deep layers of the opposite hemisphere. It has been established that callosal fibers originating in different areas of the cerebral hemispheres can have both excitatory and inhibitory effects on the same neuron.
Callosal neurons are located in different layers of the cortex, and their axonal branches come into contact in the ipsilateral hemisphere with both cortical and subcortical neurons through pyramidal tract fibers.
The diameter of callosal fibers ranges from 0.3–6.9 μm. The vast majority of fibers are less than 3 microns in diameter, and 2/3 of them are less than 1 microns. Fibers with a diameter of more than 4 microns are rare. Fibers with a diameter of 0.3 to 1 µm communicate with the slow-conducting neurons of the pyramidal tract, and fibers with a diameter of more than 1 µm communicate with a group of fast-conducting pyramidal neurons.
MT, being formed prenatally, matures and develops quite late in ontogenesis, later than other commissures of the brain. When studying the brains of infants, children and adults, it was found that myelination of the anterior commissure and MT ends only by adolescence, by 12 years ( Yakovlev, LeCours
, 1967;
Joseph
et al., 1984).
Studies of rabbit MT have shown that 45% of callosal axons are unmyelinated ( Mosidze
et al., 1977).
Most modern MRI studies in humans have confirmed that MT matures towards the end of the second decade of life ( Barkovich and Maroldo
, 1993;
Giedd
et al., 1996;
Thompson
et al., 2000).
According to other data, MT size continues to increase until the mid-twenties ( Pujol
et al., 1993).
There are studies showing that the ratio of MT size to total brain size increases during the first two decades of life ( Rauch, Jinkins
, 1994).
A significant increase in MT size on sagittal MRI images was found in children between 4 and 18 years of age ( Geers
et al., 1999).
Similar studies describe a rostral-caudal pathway of MT myelination during childhood, with an increase in the frontal region between 3 and 6 years and predominant development of the posterior part of MT between 15 and 16 years ( Thompson
et al., 2000).
Subsequent longitudinal studies showed a nonlinear increase in posterior MT by age 18 ( Geers
et al., 1999).
Considering the formation of the brain organization of mental processes in ontogenesis, A. V. Semenovich also touches on the formation of pair work of the cerebral hemispheres ( Semenovich
, 2001). The proposed model reflects three main vectors for the formation of the brain organization of mental processes: from subcortical formations to the cerebral cortex (bottom up); from the right hemisphere of the brain to the left; from the back of the brain to the front. Speaking about the formation of interhemispheric interaction associated with the maturation of brain commissures in ontogenesis, the author of the model identifies several stages. At the first stage, including the prenatal period and the first 2–3 years of life, transcortical connections at the stem level are formed, which occur within the hypothalamic-diencephalic regions and basal ganglia. In accordance with the theory of three functional blocks of the brain by A. R. Luria, at this level, interhemispheric support for the work of the first functional block of the brain occurs, which underlies the somatic, affective and cognitive status of the child. The imprinting mechanism is associated with the formation of these areas. This period ends with the fact that during the period of adaptation to speech (2–3 years), selective lateralized brainstem activation occurs, which is the key and basis for consolidating stable prerequisites for the functional lateralization of the cerebral hemispheres and the formation of hemispheric control at the following stages.
At the second stage (from 2 to 7 years), the hypocampal commissure matures, which underlies the provision of multisensory intermodal integration and memory. Interhippocampal structures play the role of initiator and stabilizer of relationships between the right and left hemispheres. In this they fundamentally differ from the commissures of the subcortical level, the main prerogative of which is the initiation of the dynamics and vector (vertical and horizontal) of interhemispheric interaction. The most important function of interhippocampal connections is the interhemispheric organization and stabilization of mnestic processes, which in this age period bear the main responsibility for ontogenesis as a whole. When these connections are disrupted, amnestic syndrome occurs. In parallel, the formation of hemispheric dominance in speech and hand occurs.
In the period from 7 to 12–15 years (third stage), a complex of transcallosal connections is formed. According to other data, the maturation of the corpus callosum occurs up to 25 years of age (see above). MT increases its controlling function and influences the underlying commissural levels, ensuring the stability of connections and functions already achieved during ontogenesis. MT makes it possible to form an interhemispheric organization of mental processes at a regulatory, indirect, voluntary level of their occurrence (that is, it provides an interhemispheric basis for the activity of the third functional block of the brain), which allows an individual to successfully adapt socially and form individual cognitive styles. “Thanks to interhemispheric interactions at this level, it is possible to consolidate the functional priority of the frontal parts of the left hemisphere...” ( Semenovich
, 2001, p. 102). This level of interhemispheric interaction is the youngest and late maturing both in ontogenesis and phylogenesis, and therefore the most vulnerable to various negative influences that can manifest themselves during brain development.
One of the important ideas in the model proposed by A. V. Semenovich, from our point of view, is the idea of the commissures of the brain as a commissural system. Speaking about consolidating the leading role of the frontal lobes of the left hemisphere at the third stage of the formation of interhemispheric interaction, the author does not specify what the “leading” is for? Maybe they are talking about the leading role of the left hemisphere in general? In this case, we return to the concept of left hemisphere dominance, even if in a weak version. In our opinion, in the context of the problem of interhemispheric interaction, the leading role of the left hemisphere of the brain (and especially its anterior sections) can be discussed in relation to the processes of developing and consolidating skills and adaptive stereotypical forms of behavior. Only in this case do the “right to left” vector and the controlling function of MT become clear.
Symptoms of agenesis of the corpus callosum
Despite the extremely low occurrence of this diagnosis, scientists have studied its symptoms well. Some of the most common manifestations of agenesis of the corpus callosum are:
- Atrophy (complete or partial) of the auditory and (or) optic nerve.
- Cystic formations in the brain tissue (porencephaly).
- Connective tissue tumors – lipomas.
- A rare disorder of intrauterine development of the fetus, schizencephaly is a cleft of the brain.
- A significant decrease in the size of the brain and skull as a whole is microencephaly.
- Multiple pathologies of the digestive system.
- Spina bifida.
- Retinal structure disorders (Ecardi syndrome).
- Early puberty.
- Delay in psychomotor development.
These and many other disorders are in one way or another closely related to the absence of the corpus callosum. As a rule, they allow a diagnosis to be made in the first 1-2 years of a child’s life. The final confirmation of the diagnosis is an MRI of the brain.
Diseases
Dysgenesis , also known as dysplasia of the corpus callosum of the brain, is a congenital pathology of the nervous structure, manifested in its abnormal development of individual areas and tissues. The disease is the result of a defect in certain chromosomes. The disease is accompanied by a violation of the tissue composition of the corpus callosum and entails a violation of its functions.
The consequences of dysgenesis of the corpus callosum of the brain manifest themselves in the form of disorders of the neurological and mental sphere of a person. These include:
- slower response to external stimuli;
- slowdown in the development of intellectual properties of the psyche;
- impairment of recognition and understanding of written speech;
- dyslexia;
- difficulty and inhibition in the processing of light signals by the brain.
In addition, there is also another pathology - the absence of the corpus callosum of the brain in a newborn - agenesis.
Agenesis
This pathology spreads to an average of 3% of the population, which is a fairly high figure. Agenesis of the corpus callosum is a disease that is often accompanied by other ailments. The congenital absence of a hemisphere-connecting structure has its own symptoms:
- Slowing down of the child’s psychological and neurological development;
- facial dysmorphism - impaired blood flow to the facial muscles;
- pathology of the gastrointestinal tract, kidneys and the presence of tumors;
- excessively rapid sexual development;
- epileptic seizures;
- gross violations of the development of internal organs;
- defects in the development of the visual system;
- diseases of the musculoskeletal system;H
Hypoplasia
This pathology is characterized by incomplete development of the tissues of the corpus callosum. Unlike the previous disease, hypoplasia is manifested by underdevelopment, and not a complete absence of structure. Hypoplasia of the corpus callosum of the brain in a child is diagnosed by doctors during the first months of life, because the manifestations of the disease are distinctive:
- spasms of unobvious origin;
- epileptic conditions (fits, local convulsions);
- faint cry of a baby;
- absence or impairment of the sensitive sphere, that is, the child may not hear, see or smell;
- weakening or lack of muscle strength, resulting in atrophy or very weak muscles.
The consequences of hypoplasia of the corpus callosum of the brain are unfavorable, and in the absence of proper diagnosis, the prognosis is unfavorable. In 70% of children with this pathology, they suffer from severe mental retardation.
Hypoplasia of the corpus callosum
Hypoplasia is a serious, but fortunately quite rare diagnosis. In essence, this, like agenesis, is a violation of the intrauterine development of brain tissue. If during agenesis the corpus callosum of the brain is completely absent, then with hopoplasia it is underdeveloped. Of course, treating this disease with modern medicine is impossible. Therapy involves a set of measures that minimize deviations in the patient’s development. Neuropsychologists recommend that patients regularly perform a specially designed set of physical exercises that help restore connections between the hemispheres, as well as information wave therapy.
Sexual dimorphism
A number of Russian and foreign scientists believe that the difference in thinking and behavioral reactions between men and women is associated with the different structure and size of the corpus callosum. Thus, Newsweek published an article explaining the nature of women's intuition: women have a slightly wider corpus callosum than men. This fact, according to the same scientists, also explains the fact that women, unlike men, are able to cope with several different tasks at the same time.
After some time, a group of French scientists reported that, as a percentage of brain size, men have a larger corpus callosum than women, but scientists did not draw any clear conclusions. Be that as it may, all scientists only agree that the corpus callosum is one of the most important structural components that performs a number of vital functions.
Recommendations
- Veluth, S; Destrieux, C; Kakou, M. (May 1998). "[Morphological anatomy of the corpus callosum]". Neuro-Surgery
.
44
(1 supplement): 17–30. PMID 9757322. - "Corpus callosum." Queensland Brain Institute
. November 10, 2021. - ^ a b
Lueders, Eileen;
Thompson, Paul M.; Toga, Arthur W. (August 18, 2010). "Development of the corpus callosum in the healthy human brain." Journal of Neuroscience
.
30
(33):10985–10990. Doi:10.1523/JNEUROSCI.5122-09.2010. PMC 3197828. PMID 20720105. - ^ a b c
Gaillard, Frank.
“Corpus callosum | Radiology Help Article | Radiopaedia.org." radiopaedia.org
. - Carpenter, Malcolm (1985). Basic text of neuroanatomy
(3rd ed.). Baltimore: Williams and Wilkins. pp. 26–32. ISBN 978-0683014556. - Cumming, W. J. (March 1970). "Anatomical overview of the corpus callosum". Cortex;
Journal dedicated to the study of the nervous system and behavior .
6
(1): 1–18. Doi:10.1016/s0010-9452 (70) 80033-8. PMID 4913253. - Ropper, A.; Samuels, M.; Klein, J. (2014). Principles of Neuroscience by Adams and Victor
(10th ed.). McGraw-Hill. item 798. ISBN 978-0071794794. - Doron, kW; Gazzaniga, M. S. (September 2008). "Neuroimaging techniques open new perspectives on callosal transfer and interhemispheric communication." Cortex;
Journal dedicated to the study of the nervous system and behavior .
44
(8):1023–9. doi:10.1016/j.cortex.2008.03.007. PMID 18672233. S2CID 5641608. - Schlaug, Gottfried; Jahnke, Lutz; Huang, Yanxiong; Staiger, Jochen F; Steinmetz, Helmut (10 April 2010). "Increased size of the corpus callosum in musicians." Neuropsychology
.
25
(4):557–577. Doi:10.1177/0743558410366594. PMID 8524453. S2CID 145178347. - Aboitiz, F (1992). "Brain connections: interhemispheric fiber systems and anatomical brain asymmetries in humans." Biological Research
.
25
(2): 51–61. PMID 1365702. - “NIAAA Publications.” pubs.niaaa.nih.gov
. - ^ a b
Caminiti, Roberto;
Ghaziri, Hasan; Galuske, Ralph; Hof, Patrick R.; Innocenti, Giorgio M. (2009). "Evolution of enhanced processing with temporally distributed slow neural connections in primates". Proceedings of the National Academy of Sciences
.
106
(46):19551–6. Bibcode:2009PNAS..10619551C. doi:10.1073/pnas.0907655106. JSTOR 25593230. PMC 2770441. PMID 19875694. - Hofer, Sabina; Frahm, Jens (2006). "Revisiting the topography of the human corpus callosum—comprehensive fiber tractography using diffusion tensor magnetic resonance imaging." NeuroImage
.
32
(3):989–94. doi:10.1016/j.neuroimage.2006.05.044. PMID 16854598. S2CID 1164423. - Davatzikos, C; Resnick, S. M. (1998). "Sex differences in anatomical measures of interhemispheric connectivity: correlations with cognition in women but not men." Cortex
.
8
(7): 635–40. Doi:10.1093/cercor/8.7.635. PMID 9823484. - Ardekani, B.A.; Figarsky, K.; Sidtis, J. J. (2012). "Sexual dimorphism of the human corpus callosum: an MRI study using the OASIS brain database." Cortex
.
23
(10): 2514–20. doi:10.1093/cercor/bhs253. PMC 3767965. PMID 22891036. - Dubb, Abraham; Gur, Ruben; Avants, Brian; Gee, James (2003). "Characteristics of sexual dimorphism of the human corpus callosum." NeuroImage
.
20
(1):512–9. Doi:10.1016/S1053-8119 (03) 00313-6. PMID 14527611. S2CID 31728989. - Westerhausen, Rene; Kreuder, Frank; Sequeira, Sara dos Santos; Walter, Christophe; Werner, Wolfgang; Wittling, Ralf Arne; Schweiger, Elizabeth; Wittling, Werner (2004). "The influence of hand and gender on the macro- and microstructure of the corpus callosum and its subregions: combined high-resolution MRI and diffusion tensor MRI." Cognitive Brain Research
.
21
(3): 418–26. doi:10.1016/j.cogbrainres.2004.07.002. PMID 15511657. - Shin, Yoon-Wook; Jin Kim, Dae; Hyun Ha, Tae; Park, Hae Jeong; Moon, Won-Jin; Chul Chung, Eun; Min Lee, Jong; Young Kim, In; Kim, Sun I.; and others. (2005). "Sex differences in the human corpus callosum: a diffusion tensor imaging study." NeuroReport
.
16
(8): 795–8. Doi:10.1097/00001756-200505310-00003. PMID 15891572. S2CID 11361577. - Contos, Despina; Megalooikonomou, Vasileios; Gee, James S. (2009). "Morphometric analysis of brain images with a reduced number of statistical tests: a study of gender differentiation of the corpus callosum." Artificial Intelligence in Medicine
.
47
(1):75–86. Doi:10.1016/j.artmed.2009.05.007. PMC 2732126. PMID 19559582. - Spasojevic, Goran; Stojanovic, Zlatan; Suscevic, Dusan; Malobabic, Slobodan (2006). "Sexual dimorphism of the human corpus callosum: a digital morphometric study." Vojnosanitetski Pregled
.
63
(11):933–8. Doi:10.2298/VSP0611933S. PMID 17144427. - Yokota, Y.; Kawamura, Y.; Cameo, Y. (2005). Shapes of the corpus callosum in the midsagittal plane: MRI differences in normal men, normal women, and RHI
.
2005 IEEE Engineering in Medicine and Biology, 27th Annual Conference
.
3
. pp. 3055–8. Doi:10.1109/IEMBS.2005.1617119. ISBN 978-0-7803-8741-6. PMID 17282888. S2CID 351426. - ^ a b
Witelson, S. (1985).
"Brain connection: The corpus callosum is larger in left-handed people." The science
.
229
(4714): 665–8. Bibcode:1985Sci … 229..665 W. Doi:10.1126/science.4023705. PMID 4023705. - Driesen, Naomi R.; Raz, Naftali (1995). "Effects of gender, age and hand on morphology of the corpus callosum: a meta-analysis". Psychobiology
.
23
(3): 240–7. - Lueders, Eileen; Cherbuin, Nicholas; Thompson, Paul M.; Gutman, Boris; Anstey, Kaarin J.; Sachdev, Perminder; Toga, Arthur W. (2010-08-01). "When more is less: the relationship between corpus callosum size and hand lateralization." NeuroImage
.
52
(1):43–49. doi:10.1016/j.neuroimage.2010.04.016. ISSN 1053-8119. PMC 2903194. PMID 20394828. - Clark, Dave F.; Welless, James W.; Chacon, Monica M.; Breuer, Joshua; Koenig, Mary Kay; McManis, Mark; Castillo, Edward; Baumgartner, James E. (2007). "Body callosotomy: a palliative therapeutic modality may help identify resectable epileptogenic lesions." Capture
.
16
(6): 545–53. doi:10.1016/j.seizure.2007.04.004. PMID 17521926. S2CID 18192521. - "WebMd Corpus Callotomy". Web MD. July 18, 2010. Archived from the original July 2, 2010. Retrieved July 18, 2010.
- Rakic, P; Yakovlev P.I. (January 1968). "Development of the corpus callosum and septum of the corpus cava in humans." Journal of Comparative Neuroscience
.
132
(1):45–72. Doi:10.1002/cne.901320103. PMID 5293999. - Rash, BG; Richards, L. J. (28 May 2001). "The role of pioneer axons of the cingulate gyrus in the development of the corpus callosum." Journal of Comparative Neuroscience
.
434
(2):147–57. Doi:10.1002/cne.1170. PMID 11331522. - Dobyns, W. B. (1996). “Absence increases search time.” American Journal of Human Genetics
.
58
(1): 7–16. PMC 1914936. PMID 8554070. - "NINDS Agenesis of the Callosum Information Page: NINDS." RightDiagnosis.com
. In the archive from the original dated March 24, 2012. Retrieved August 30, 2011. - Wegiel, Jarek; Kaczmarski, Wojciech; Flory, Michael; Martinez-Cerdeno, Veronica; Wisniewski, Thomas; Nowicki, Krzysztof; Kuchna, Isabela; Wegiel, Jerzy (December 19, 2021). “Corpus callosal axonal deficits, reduced axonal diameter, and reduced area are markers of abnormal development of interhemispheric connections in autistic subjects.” Acta Neuropathologica Communications
.
6
. Doi:10.1186/s40478-018-0645-7. ISSN 2051-5960. PMC 6299595. PMID 30567587. - "Autism may cause a lack of connectivity and coordination in certain areas of the brain, researchers have found." Medical news today
. In the archive from the original dated 10/15/2011. - ^ a b
Bishop, Catherine M.;
Wallsten, Douglas (1997). "Sex differences in the human corpus callosum: myth or reality?" (PDF). Neuroscience and Biobehavioral Reviews
.
21
(5):581–601. Doi:10.1016/S0149-7634 (96) 00049-8. PMID 9353793. S2CID 9909395. - Delacoste-Uthamsing, K; Holloway, R. (1982). "Sexual dimorphism of the human corpus callosum". The science
.
216
(4553):1431–2. Bibcode:1982Scientific ... 216.1431D. Doi:10.1126/science.7089533. PMID 7089533. - S. Gorman (20 January 1992). "Gender Assessment". Time
: 36–43. Cited by Bishop and Wallsten. - Lueders, Eileen; Narr, Catherine L.; Seidel, Eran; Thompson, Paul M.; Toga, Arthur W. (2006). "Gender effects on callosal thickness in scaled and unscaled space." NeuroReport
.
17
(11): 1103–6. Doi:10.1097/01.wnr.0000227987.77304.cc. PMID 16837835. S2CID 14466914. - Keeler, Clyde E. (1933). "Absence of the corpus callosum as a Mendelian character in the house mouse". Proceedings of the National Academy of Sciences of the United States of America
.
19
(6): 609–11. Bibcode:1933PNAS ... 19..609K. Doi:10.1073/psas.19.6.609. JSTOR 86284. PMC 1086100. PMID 16587795. - Sarnat, Harvey B. and Paolo Curatolo (2007). Malformations of the nervous system: Handbook of clinical neurology
, paragraph 68 - Ashwell, Ken (2010). Neurobiology of Australian marsupials: brain evolution under radiation from other mammals
, paragraph 50 - Armati, Patricia J., Chris R. Dickman, and Ian D. Hume (2006). Marsupials
, item 175 - Butler, Anne B. and William Hodos (2005). Comparative Neuroanatomy of Vertebrates: Evolution and Adaptation
, paragraph 361 - Morris, H., & Schaeffer, J. P. (1953). The nervous system is the brain or encephalon. Human anatomy; a complete systematic treatise. (11th ed., pp. 920–921, 964–965). New York: Blackiston.