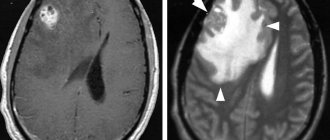
Hypertensive encephalopathy
Hypertensive encephalopathy is a complication of severe hypertension (often fatal) that develops when blood pressure levels exceed the body's ability to regulate and maintain a stable blood supply to the brain. That is, the vessels of the brain cannot withstand the pressure of blood under high pressure. Factors contributing to the development of acute encephalopathy are varied - cessation of blood pressure-lowering medications, severe emotional stress, surgery and anesthesia, acute fluid load with an increase in circulating blood volume, preeclampsia, acute renal failure, etc.
If it is not possible to quickly lower blood pressure immediately after the appearance of signs of hypertensive encephalopathy, coma and death inevitably occur. The course of hypertensive encephalopathy is sometimes complicated by intracranial bleeding, cerebral infarction, heart failure or myocardial ischemia.
Rapidly lowering blood pressure completely eliminates the symptoms of hypertensive encephalopathy and reduces the risk of catastrophic damage to the brain or cardiovascular system.
The patient's blood pressure against the background of hypertensive encephalopathy may be relatively low (for example, 160/100 -170/110 mmHg). However, if it exceeds the individual upper threshold of autoregulation of cerebral blood flow, then this acute complication of hypertensive crisis develops.
Hypertensive encephalopathy is usually observed in children with acute glomerulonephritis, in young women with eclampsia, and also with an overdose of drugs that stimulate the release of excitatory hormones into the blood. In patients with chronic high blood pressure, this complication is observed less frequently - it develops in them, as a rule, only with very high blood pressure (250/150 mm Hg and above), which is associated with adaptation of the processes of regulating blood circulation in the vessels of the brain to constantly elevated blood pressure.
Symptoms of hypertensive encephalopathy: headache, nausea, vomiting, visual disturbances (diplopia, nystagmus) appear first. If treatment is not started at this time, neurological symptoms develop: disorientation, paresis, aphasia, confusion, focal or generalized convulsions, followed by coma and death. The increase in symptoms occurs gradually, over 24-48 hours, which helps to differentiate hypertensive encephalopathy from intracerebral hemorrhage, the symptoms of which develop suddenly and rapidly.
The diagnosis of hypertensive encephalopathy is established by excluding stroke, tumor, encephalitis and some other brain diseases. A distinctive feature of acute hypertensive encephalopathy is its complete reversibility provided that treatment is started in a timely manner, when the pressure can be reduced before the development of severe cerebral edema with herniation of the cerebellum into the foramen magnum.
Thus, rapid improvement in the patient’s condition after initiation of therapy can serve as a criterion for diagnosing hypertensive encephalopathy. Indeed, during a stroke, a decrease in blood pressure, on the contrary, worsens the patient’s condition and intensifies the damage. A preliminary diagnosis is made based on the medical history (if possible), the nature and dynamics of symptoms and examination data of the patient.
Perinatal encephalopathy - symptoms and treatment
Treatment of perinatal pathology in the acute period is carried out in the neonatology department.
Surgical treatment may be necessary if the child has a large hematoma inside the cranial cavity; its removal is only possible through surgery.
With increasing closed hydrocephalus, in order to avoid brain atrophy from compression by fluid, children undergo shunt surgery - a shunt is installed (a plastic tube through which excess fluid from the cranial cavity is drained into the abdominal cavity and absorbed there).
However, most parents are faced with the need to treat a child with perinatal encephalopathy during the recovery period of up to 1 year due to impaired muscle tone, developmental delay, hypertensive-hydrocephalic syndrome and other manifestations.
The greatest effect can be achieved using an integrated treatment approach.
Therapeutic medical massage is carried out to normalize muscle tone and improve trophic processes in tissues. Should only be performed by a massage therapist with medical training and experience working with children with perinatal brain damage. The procedure is carried out in children's clinics and rehabilitation centers [3][5][7].
Exercise therapy - physical therapy. May include various types:
- Vojta therapy;
- Bobat-terpiya;
- therapeutic exercises on balls;
- mechanotherapy (exercise exercises on a simulator);
- classes with an instructor in the swimming pool.
Exercise therapy should only be carried out by an instructor with special education [6][7][18].
Microcurrent reflexology (MCRT) is a new medical technology approved by the Ministry of Health of the Russian Federation and recommended for the treatment of children with organic brain damage, delayed motor, speech, mental development and cerebral palsy. Treatment is carried out on an outpatient basis in rehabilitation centers in various regions of the Russian Federation.
The therapeutic effect is physiological and painless, using currents in the microampere range on neuroreflex zones in various areas of the skin. Microcurrents are 10 times less than with standard physiotherapy. Treatment is carried out according to an individual scheme, taking into account all the manifestations of perinatal encephalopathy present in the child.
During the treatment process, normal reflex activity of the brain is restored, muscle tone is normalized: spastic (tense) muscles are relaxed, hypotonic (weak) muscles are stimulated. MPRT stabilizes cerebral vascular tone, which allows compensation of intracranial pressure.
The method promotes the acquisition of new motor skills by restoring the activity of neurons in various areas of the cerebral cortex and cerebellum affected by hypoxia and hydrocephalus [4][6][9][14][15].
Medicines of the nootropic group (Cortexin, Pantogam, Ceraxon, etc.) should be prescribed only by a pediatric neurologist strictly according to indications, i.e., if the child has delayed psychomotor development and there are no contraindications [11][16].
diuretics only when intracranial pressure increases (expansion of the cerebrospinal fluid spaces on the NSG, the presence of clinical manifestations of hypertensive-hydrocephalic syndrome).
Pediatric neurologists, as a rule, prescribe the diuretic drug Diacarb, which also has an additional effect on the choroid plexuses of the brain - it reduces excess formation of cerebrospinal fluid and reduces intracranial pressure. However, it should be used only in combination with Asparkam, since the use of this diuretic leads to the loss of such a trace element as potassium, Asparkam replenishes its loss [16]. In mild cases in infancy, it is advisable to use gentle herbal preparations: fennel tea, dill water, approved for use in childhood.
Arterial hypertension (AH) occupies one of the leading places in the structure of morbidity and mortality. According to WHO, hypertension affects from 20 to 40% of the population of developed countries. Russia is one of the countries with a very high prevalence of hypertension: in the mid-1990s. this figure was 39.9% among men and 41.1% among women [1]. The results of monitoring the epidemiological situation with hypertension, carried out within the framework of the targeted federal program “Prevention, diagnosis and treatment of hypertension in Russia”, showed that over the past 10 years the situation has remained virtually unchanged: in 2004, the prevalence of hypertension was still 36.9% among men and 42% among women [2]. In addition, the results of the work demonstrate an increase in the prevalence of hypertension with age: after 60 years it reaches 60%, and after 80 it approaches 80%. In 95% of cases we are talking about essential hypertension, while symptomatic hypertension is detected only in 5% of patients. Factors influencing the development of hypertension are presented in the table.
. Factors influencing the development of hypertension.
Considering the external and internal factors responsible for the development of hypertension, it is possible to formulate directions for preventive and therapeutic effects on both hypertension and its complications. Impact on external factors is possible only if the patient is motivated to fight hypertension. Unfortunately, the ability and desire of patients, especially men, to change their lifestyle to eliminate the main external factors influencing hypertension is extremely low [3]. Both in Russia and in the world, the number of patients who control blood pressure (BP) is small: but if in France, Greece, Germany, Spain and the USA the target blood pressure level is achieved in 30.0–35.7% of patients with hypertension, then in In Canada, the Czech Republic, Poland, India and Russia, this value does not exceed 9–16% [4].
There are many reasons for low adherence of patients to antihypertensive therapy: • poor tolerability of antihypertensive drugs; • low discipline of patients; • high cost of medicines; • psychological factors (reluctance to take medications for life); • lack of awareness about the complications of hypertension and the purposes of taking medications; • existing cognitive deficit – memory loss (patients forget to take medication), absent-mindedness (irregularity of taking medications), decreased criticism of their condition; • antihypertensive drugs do not eliminate many neurological symptoms (dizziness, headache, unsteadiness when walking, anxiety, irritability) characteristic of brain damage in hypertension.
Systematic violations of antihypertensive therapy or refusal of it lead to a decrease in the effectiveness of treatment and worsening hypertension with the development of complications from target organs [5].
As a result of hypertension, damage occurs to the heart, blood vessels, kidneys, and brain. One of the most dangerous complications of hypertension is acute cerebrovascular accident. Both new and repeated strokes are the direct cause of death of the patient in 57% of cases in the early stages and in 14% in the long-term period [6]. Also very acute in modern neurology is the issue of chronic brain damage due to hypertension, in particular hypertensive encephalopathy (HE). This term dates back to the late 1950s. by the staff of the Institute of Neurology G.A. Maksudov. and Schmidt E.V. and is defined as a steadily progressive lesion of the brain substance, which is associated with a poorly controlled increase in blood pressure, leading to chronic cerebral ischemia.
Among the risk factors for the development of GE are the following: uncontrolled hypertension, hypertensive crises, high blood pressure variability, high nocturnal hypertension, excessive decrease in blood pressure, including iatrogenic ones [7].
Three stages can be distinguished during GE. At stage I, the clinic is dominated by subjective disorders in the form of general weakness and fatigue, emotional lability, sleep disturbances, decreased memory and attention, and headaches. Neurological symptoms are not formed by distinct neurological syndromes, but are represented by anisoreflexia, discoordination, and symptoms of oral automatism. Violations of memory, praxis and gnosis can, as a rule, be identified only when special tests are carried out.
At stage II, there are more subjective complaints, and neurological symptoms can already be divided into distinct syndromes (pyramidal, discoordination, amyostatic, dysmnestic), with one neurological syndrome usually dominating. Professional and social adaptation of patients decreases. At stage III, neurological symptoms increase, a distinct pseudobulbar syndrome appears, and sometimes paroxysmal conditions (including epileptic seizures); Severe cognitive impairment leads to disruption of social and everyday adaptation and complete loss of working capacity. Thus, GE ultimately leads to the formation of vascular dementia, and its subcortical variant is often formed [18].
In stages II and III of GE, diffuse changes in the brain substance are usually combined with focal lesions in the form of lacunar infarcts - small cavities ranging in size from 0.1 to 1.0 cm, formed in areas of cerebral ischemia. In the structure of cerebrovascular diseases, there is an increase in the proportion of lacunar strokes: among all cases of stroke in hypertension they account for 15%. It is possible either asymptomatic development of lacunar infarction, if the foci are located in functionally silent areas, or the formation of a transient ischemic attack, stroke. Lacunar infarcts are localized in the basal ganglia, internal capsule, thalamus, pons, cerebellum and white matter of the hemispheres. The formation of multiple small-focal brain lesions - lacunar state - significantly worsens the prognosis of the course of GE and reduces the possibility of drug improvement of the patient's condition - even when good control of hypertension is achieved. In addition, lacunar strokes are risk factors for the development of hemorrhagic stroke and vascular dementia [19].
With hypertension, the following pathomorphological changes occur in the vessels of the brain: plasmatic and hemorrhagic impregnation, necrosis of the vascular wall with its subsequent thinning, adaptive thickening of the walls of extracerebral vessels. In GE, early damage to the predominantly white matter of the brain is observed, which is the destruction of the myelin of the central conductors, which has a typical picture in computed tomography (decreased signal intensity) and magnetic resonance imaging (increased signal intensity) [8]. Characteristic changes are usually detected in areas of the terminal blood supply that are particularly sensitive to fluctuations in blood pressure, i.e., in the periventricular areas of the brain, and are called leukoaraiosis [9, 10]. With uncontrolled hypertension, the described processes in the white matter progress, the phenomenon of corticocortical disconnection develops (conductive fibers are localized in the white matter), intellectual-mnestic functions are disrupted, and ultimately vascular dementia is formed (“subcortical arteriolosclerotic encephalopathy”, or Binswanger’s disease). The ARIC study showed that such changes in the white matter of the brain occur in the group of patients with hypertension 2 times more often than in normotensive patients, and in patients with uncontrolled hypertension compared to patients with adequate control of hypertension - 1.5 times more often. Often such changes occur without clinical symptoms. The duration of the asymptomatic phase may vary and is determined by the presence of other risk factors [11].
With hypertension, atherosclerosis rapidly progresses. The structural and functional properties of erythrocytes and platelets are disrupted - their ability to deform worsens, hematocrit increases, blood viscosity increases, which in turn leads to disruption of microcirculation. Pathomorphological, as well as isolated clinical studies of the cerebral venous system in hypertension indicate severe disturbances, including obliteration of the cerebral venous sinuses. In this case, they speak of encephalopathy of mixed origin: hypertensive and atherosclerotic [12, 13].
An important consequence of changes in the structure of the vascular wall is a violation of the autoregulation of cerebral circulation - the ability to maintain stable blood flow when the average systemic blood pressure fluctuates from 60 to 150-170 mm Hg. Art. [14]. Experimental data indicate that autoregulation disorders are primarily observed in the white matter of the brain and, to a lesser extent, in the cerebral cortex [15]. As a result, hypoxia develops, caused by a discrepancy between the tissue need for oxygen and its delivery.
At the initial stage of oxygen starvation in mitochondria, the rate of aerobic oxidation and oxidative phosphorylation decreases, which leads to a decrease in protein synthesis and gene expression, a decrease in the amount of adenosine triphosphate (ATP), and an increase in the content of adenosine diphosphate (ADP) and adenosine monophosphate. With a further decrease in cerebral blood flow, the enzyme phosphofructokinase is activated, anaerobic glycolysis is enhanced, and then the final transition to anaerobic respiration occurs, which adapts the cell to hypoxia, but glycogen reserves are depleted. This, in turn, entails the accumulation of under-oxidized lactate with the development of lactic acidosis. At the same time, the activity of lactate dehydrogenase increases and the activity of succinate dehydrogenase, which supplies electrons to the respiratory chain of mitochondria, decreases, which indicates a disruption in the processes of energy formation in the ischemic brain. Under such conditions, anaerobic glycolysis does not occur, which leads to severe energy deficiency. Ultimately, destabilization of cell membranes occurs, disruption of ion channels, damage to the potassium-sodium pump, which leads to cell hyperhydration, cloudy swelling, and then to balloon degeneration. The most important role in this process belongs to glutamate receptors.
Oxidative stress, closely associated with the ischemic cascade, occurs when glutamate receptors are excited and consists of excessive accumulation of free radicals, activation of lipid peroxidation and excessive intracellular accumulation of their products. The reactions of oxidative stress and the ischemic cascade interact and potentiate each other.
Free radicals are highly reactive forms of oxygen, hydrogen peroxide, and aldehydes formed under hypoxic conditions. Under their influence, the functional properties of a number of enzymes, carbohydrates, proteins, including DNA and RNA, change; as a result, the cell loses its functions, abnormal proteins appear and secondary destructive processes are stimulated [16–18].
Along with the processes of free radical oxidation, stable antioxidant radicals or antioxidants are produced in biological objects. The mechanism of their action is based on inhibition of free radical processes in tissues, which inhibits the development of destructive changes and inactivates oxidative stress reactions. Changes in the structure and function of substrates under conditions of ischemia and stress depend on the ratio of the activity of free radicals and antioxidants.
The body has a physiological antioxidant system that maintains oxidative-antioxidant balance both in liquid media (blood, lymph, intracellular and intercellular fluid) and in the structural elements of the cell (plasmic, endoplasmic, mitochondrial, cell membranes). Enzymatic antioxidants include: superoxide dismutase, catalase, glutathione dehydroascorbate reductase, and some other peroxidases.
Non-enzymatic antioxidants include vitamins C, E, K, glucose, ubiquinones, phenylalanine, transferrin, haptoglobin, tryptophan, ceruloplasmin, carotenoids. Biological and chemically synthesized antioxidants are divided into fat- and water-soluble. The former are localized where the target substrates for attack by free radicals and peroxides are located, the biological structures most vulnerable to peroxidation processes, which include primarily biological membranes and blood lipoproteins (their main targets are unsaturated fatty acids). The most significant fat-soluble antioxidant is α-tocopherol. Among the water-soluble antioxidants, the most important are glutathione and the ascorbic acid system, which are especially significant for the antioxidant protection of the brain [21, 22].
At all stages of GE, to the treatment of the underlying disease - hypertension - it is necessary to add drugs that improve cerebral blood flow and metabolism of nervous tissue, as well as those acting on internal factors that can affect the progression of encephalopathy. In order to prevent the increase in the atherosclerotic process, it is necessary to normalize fat metabolism: reducing body mass index, low-fat diet, prescribing statins (simvastatin, pravastatin). In order to improve the rheological properties of blood, antiplatelet agents (acetylsalicylic acid, ticlopedine, clopidogrel, dipyridamole) are indicated. The CAPRIE and ESPS-2 studies showed that the use of acetylsalicylic acid, clopidogrel and dipyridamole reduces the risk of developing cerebral ischemia [20]. At all stages of GE, the use of drugs that act on the vascular wall, improve the metabolism of brain tissue and have neuroprotective properties is mandatory.
Based on the pathogenetic role of hypoxia and oxidative stress, the use of antioxidants, which are specific correctors of brain energy metabolism, is considered as one of the most promising methods of treating chronic forms of cerebral circulatory disorders, including HE.
Currently, α-tocopherol, ascorbic acid, methionine, cerulloplasmin, carotene, ubiquinone, and emoxypine are used in clinical practice. However, the disadvantage of these drugs is the need for long-term use (several weeks) to ultimately achieve weak antioxidant and antihypoxic effects. This provided the prerequisites for the creation of more effective drugs with antioxidant properties, among which Mexidol® occupies a prominent place [19].
Mexidol is a derivative of 3-hydroxypyridine, which belongs to the water-soluble antioxidants of the biogenic type and is a structural analogue of compounds of the vitamin B6 group. An important property of this drug is its ability to penetrate the blood-brain barrier. The drug Mexidol (2-ethyl6-methyl-3-hydroxypyridine succinate) was created on the basis of emoxypine with the inclusion of succinic acid in its molecule.
In recent years, the effect of succinic acid, its salts and esters, which are universal intracellular metabolites, has been widely studied. Succinic acid is found in all tissues and organs. Performing a catalytic function in relation to the Krebs cycle, this compound reduces the concentration of other products of the cycle in the blood - lactate, pyruvate, citrate, produced and accumulated in the early stages of hypoxia, and is thereby included in energy metabolism, directing the oxidation process along the most economical path. The Roberts cycle functions in nervous tissue, during which succinic acid is formed from γ-aminobutyric acid (GABA) through the intermediate stage of succinic aldehyde. The formation of succinic acid is also possible under conditions of hypoxia and oxidative stress in the reaction of oxidative deamination of α-ketaglutaric acid in the liver. The antioxidant effect of succinic acid is associated with its effect on the transport of mediator amino acids, as well as with an increase in the content of aminobutyric acid in the brain due to the Roberts shunt. Succinic acid in the body normalizes the content of inflammatory mediators histamine and serotonin, increases microcirculation in organs and tissues, primarily in the brain, without affecting blood pressure and heart function. The antihypoxic effect of succinic acid is associated with the activation of succinate dehydrogenase oxidation and restoration of the activity of cytochrome oxidase, the key redox enzyme of the respiratory chain.
Accordingly, based on the chemical formula, Mexidol is an antioxidant, membrane protector, antihypoxant with direct energizing action, inhibiting free radicals, reducing the activation of lipid peroxidation, increasing the activity of its own physiological antioxidant system, activating the energy-synthesizing functions of mitochondria and improving energy metabolism in the cell. Mexidol has a modulating effect on membrane-bound enzymes, ion channels, receptor complexes, including GABA and acetylcholine, improves synaptic transmission in brain structures, correcting disorders in microcirculatory systems. Mexidol acts under conditions of ischemia and hypoxia as a specific trap of free radicals, reducing their damaging effect on cerebral structures [23]. The drug is prescribed in doses of 200 to 500 mg 2-4 times a day intravenously in a stream or drip with saline or intramuscularly. The maximum daily dose is 1200 mg. Then it is possible to switch to the tablet form of administration. Mexidol tablet contains 125 mg of ethylmethylhydroxypyridine succinate; The drug is prescribed orally at 125–250 mg 3 times a day. Treatment begins with a dose of 125–250 mg 1–2 times a day with a gradual increase until a therapeutic effect is obtained. The duration of treatment is usually 2–6 weeks. Treatment is stopped gradually, reducing the dose over 2-3 days.
In patients with HE, long-term planned therapy with antioxidants significantly improves the quality of life and helps prevent the progression of functional-morphological cerebral disorders. Early therapy with antioxidants is currently considered as a pathogenetically determined method for correcting cerebral metabolism in hypertensive encephalopathy.
GE is a severe progressive disease that forms a variety of neurological syndromes, threatens the development of strokes and leads to vascular dementia. Timely treatment can preserve the patient’s professional, social and everyday adaptation for many years, and improves the prognosis for the patient’s life expectancy.