1.What are extrapyramidal disorders?
Extrapyramidal disorders
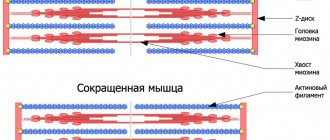
represent disturbances in muscle tone, which affects motor activity.
Movements can be obsessive, uncontrollable, or, conversely, impossible (although previously they did not present any difficulties). The severity of these disorders varies from small tics and paresis to constant trembling or obsessive voluntary contraction of a muscle group.
A must read! Help with treatment and hospitalization!
Psychiatry Psychiatry and psychopharmacotherapy named after. P.B. Gannushkina No. 06 2001
Neuroleptics, or antipsychotics, constitute one of the main groups of modern psychotropic drugs and occupy a central place in clinical psychopharmacology. Antipsychotic drugs include drugs of various chemical groups that have a number of common psychopharmacological properties. These properties were most fully formulated by J. Delay and P. Deniker (1961): 1) psycholeptic effect without a hypnotic effect; 2) a relieving effect in relation to various types of excitement, including manic; 3) a reducing effect on some acute, chronic and experimental psychoses; 4) the ability to cause characteristic neurological and autonomic disorders; 5) predominant effect on the subcortical structures of the central nervous system.
Later, A. Carlsson et al.
(1963–1987) showed that the peculiarities of the clinical action of antipsychotics are due to their general neurochemical properties, which consist in the ability to selectively block dopamine D2 receptors in various parts of the brain (striatum, nucleus accumbens, cerebral cortex) with suppression of central dopaminergic transmission and a compensatory increase in the rate of biosynthesis developing and dopamine metabolism in the corresponding brain structures. The development of side neurological effects of classical neuroleptics is associated with blockade of dopamine D2 receptors in the nigrostriatal system of the brain. The main difference between the new generation of antipsychotics (Rispolept, Zyprexa, etc.) is that they do not cause or almost do not cause extrapyramidal side effects. This property is determined by the spectrum of their neurochemical activity. Atypical antipsychotics have selectivity of action in relation to the mesolimbic and mesocortical dopaminergic system of the brain and a less pronounced effect on the nigrostriatal system. In addition, atypical antipsychotics, in addition to blocking dopamine receptors, simultaneously block 5HT2-serotonin receptors. The relationship between the blockade of dopamine and serotonin receptors plays an important role in the mechanism of action of atypical antipsychotics (R. Baldessarini, 1987; H. Meltzer, 1989; F. Bamaster et al., 1997). It has been established that blockade of 5HT2-serotonin receptors leads to a compensatory increase in the concentration of dopamine in the nigrostriatal system and thereby reduces the severity of extrapyramidal side effects caused by the dopamine-blocking activity of these antipsychotics (C. Saller et al., 1990). According to the American DSM-IV classification, all extrapyramidal movement disorders associated with taking antipsychotics can be divided into parkinsonism, acute dystonia, acute akathisia
and
tardive dyskinesia
.
The diagnostic criteria for extrapyramidal neuroleptic disorders according to DSM-IV are presented in table. 1. Table 1.
Diagnosis criteria for antipsychotic-induced parkinsonism according to DSM-IV
A. The presence of one or more symptoms that appear during neuroleptic therapy: 1) tremor (extremities, head, tongue) 2) muscle rigidity with a plastic increase in muscle tone and the “cogwheel” symptom
3) akinesia B. Symptoms of group A appear within the first few weeks after the start of antipsychotic therapy or an increase in the dose of previously taken antipsychotics and decrease after the appointment of antiparkinsonian correctors C. Symptoms of group A should not be caused by a mental illness (catatonia, negative symptoms of schizophrenia, motor lethargy during a major depressive episode, etc.) D. Group A symptoms should not be caused by other drugs, neurological or somatic diseases (Parkinson's disease, Wilson's disease, etc.)
Criteria for diagnosing acute dystonia (acute dyskinesia) caused by taking antipsychotics, according to DSM-IV
A. The presence of one or more symptoms that appear during neuroleptic therapy: 1) incorrect position of the head and neck in relation to the body (retrocollis, torticollis) 2) spasms of the masticatory muscles (trismus, yawning, grimacing) 3) impaired swallowing (dysphagia), speech or breathing (laryngo-pharyngeal spasms, dysphonia) 4) slurred and difficult speech caused by hypertonicity or enlargement of the tongue (dysarthria, macroglossia) 5) protruding tongue 6) spasms of the extraocular muscles (oculogyric crises) B. Symptoms of group A develop during the first 7 days from the start of antipsychotic therapy or increasing the dose of previously taken antipsychotics and decrease after the appointment of antiparkinsonian correctors C. Symptoms of group A should not be caused by a mental illness (for example, catatonic form of schizophrenia) D. Symptoms of group A should not be caused by other drugs, neurological or somatic illness
Criteria for diagnosing acute akathisia caused by taking antipsychotics according to DSM-IV
A. The appearance of subjective complaints of anxiety after the prescription of antipsychotics B. The presence of one of the following symptoms: 1) fidgetiness, swaying 2) stomping from foot to foot 3) constant walking to relieve anxiety 4) inability to sit or stand quietly for several minutes C Symptoms of groups A and B appear within the first 4 weeks from the start of antipsychotic therapy or an increase in the dose of antipsychotics and decrease after the appointment of antiparkinsonian correctors D. Symptoms of groups A and B should not be caused by a mental illness (psychomotor agitation in schizophrenia, agitated depression, mania , hyperactivity syndrome and other conditions) E. Symptoms of groups A and B should not be caused by other drugs, neurological or somatic disease
Diagnostic criteria for neuroleptic tardive dyskinesia according to DSM-IV
A. Involuntary movements of the tongue, jaw, trunk, limbs, arising in connection with the prescription of antipsychotics B. Involuntary movements are observed for at least 4 weeks and are characterized by the following manifestations: 1) choreiform movements 2) athetoid movements 3) rhythmic movements (stereotypes) C. Symptoms of groups A and B occur during antipsychotic therapy or within 4 weeks after discontinuation of conventional and 8 weeks after discontinuation of deponeuroleptics D. The duration of treatment with antipsychotics should be at least 3 months (1 month if the patient is 60 years of age or older) E Symptoms of groups A and B should not be caused by a neurological or any general somatic disease (Huntington's chorea, Wilson's disease, Sydenham's chorea and other diseases), as well as the prescription of other drugs (L-dopa, bromocriptine) F. Symptoms of groups A and B should not be a manifestation of acute neuroleptic dystonia (acute dyskinesia)
Different antipsychotics have different extrapyramidal activity. It has been established that the ability of classical antipsychotics to cause extrapyramidal disorders increases with a known pattern from aliphatic to piperazine phenothiazine derivatives and to butyrophenones. At the same time, the nature of side extrapyramidal effects changes - from the predominant akinetic-rigid syndrome to hyperkinetic and dyskinetic syndrome (G.Ya. Avrutsky, I.Ya. Gurovich, V.V. Gromova, 1974 ). Atypical antipsychotics have a dose-dependent effect on the development of extrapyramidal disorders.
At moderate therapeutic doses, Rispolept and Zyprexa cause extrapyramidal symptoms with the same frequency as placebo.
As dosages increase (Rispolept more than 6 mg/day and Zyprexa more than 10 mg/day), the incidence of extrapyramidal side effects exceeds placebo, but is significantly lower than haloperidol (J. Peuskens, 1992; F. Muller-Spahn, 1992; D. Casey, 1997). An exception, according to our data, may be patients with schizophrenia with symptoms of residual cerebral organic failure, in whom even low doses of drugs can cause the appearance of extrapyramidal disorders. Comparative characteristics of extrapyramidal side effects of antipsychotics are presented in Table. 2. Neuroleptic parkinsonism
occurs in more than 50% of cases when treated with classical antipsychotics during the first weeks from the start of antipsychotic therapy or increasing the dose of previously taken antipsychotics and is characterized by the appearance of general stiffness with a characteristic posture with elbows bent and arms brought to the body, tremor of the limbs , akathisia and accompanying autonomic disorders (facial greasiness, sweating, seborrhea).
Muscle tone is increased according to the plastic type with the “cogwheel” symptom. Various hyperkinesis may be observed, which are not persistent. Treatment.
As a rule, extrapyramidal symptoms are reduced after the prescription of antiparkinsonian correctors - cyclodol, akinetone, etc. (
Table 3
).
In patients with residual cerebral organic insufficiency, extrapyramidal symptoms can acquire a protracted course - “prolonged extrapyramidal syndrome” according to I.Ya. Gurovich (1971). In these cases, high doses of antiparkinsonian correctors are prescribed in combination with nootropics, the dose of antipsychotics taken is reduced, and drugs with minimal extrapyramidal activity are prescribed ( see Table 2
).
Our studies have shown that in severe, protracted extrapyramidal neuroleptic syndrome, extracorporeal detoxification methods - plasmapheresis
and
hemosorption -
.
Table 2. Comparative characteristics of extrapyramidal side effects of antipsychotics (according to the literature and our own experience in using the drugs)
| A drug | Parkinsonism | Hyperkinetic manifestations (tremor, hyperkinesis, akathisia) | Dyskinesia |
| Aliphatic phenothiazine derivatives | |||
| Aminazine (chlorpromazine) | ++ | + | + |
| Propazine (promazine) | + | + | +- |
| Tizercin (levomepromazine) | + | + | — |
| Teralen (alimemazine) | + | + | +- |
| Piperazine phenothiazine derivatives | |||
| Frenolone (metofenazine) | + | + | + |
| Etaperazine (perphenazine) | ++ | + | + |
| Triftazine (trifluoperazine) | ++ | ++ | ++ |
| Majeptyl (thioproperazine) | ++ | +++ | +++ |
| Moditene (fluorophenazine) | ++ | +++ | ++ |
| Metherazine (prochlorperazine) | ++ | ++ | ++ |
| Piperedine phenothiazine derivatives | |||
| Sonapax (thioridazine) | + | +- | +- |
| Neuleptil (periciazine) | ++ | + | + |
| Piportil (pipothiazine) | ++ | ++ | ++ |
| Butyrophenone derivatives | |||
| Haloperidol | ++ | ++ | ++ |
| Trisedyl (trifluperidol) | +++ | +++ | ++ |
| Diphenylbutylpiperidine derivatives | |||
| Orap (pimozide)* | + | ++ | + |
| Imap (flushpirelen)* | + | + | ++ |
| Semap (penfluridol) | + | ++ | + |
| Dibenzodiazepine derivatives | |||
| Leponex (clozapine)** | +- | +- | — |
| Olanzapine (Zyprexa)** | +- | + | +- |
| Thioxanthene derivatives | |||
| Chlorprothixene (Truxal) | + | — | — |
| Clopixol (zuclopenthixol) | ++ | + | + |
| Fluanxol (flupenthixol) | ++ | + | + |
| Thiothixene (navan) | ++ | ++ | ++ |
| Substituted benzamides | |||
| Eglonil (sulpiride) | +- | + | — |
| Topral (sultopride) | ++ | ++ | + |
| Tiapride (tiapridal) | + | + | + |
| Benzisoxazole derivatives | |||
| Risperidone (Risperdal)** | +- | + | +- |
| Note. The sign (+) indicates the approximate severity of the effect when using the drug in average therapeutic doses; sign (-) - no effect; * - the drugs are currently discontinued; ** - atypical antipsychotics practically do not cause the development of extrapyramidal side effects and do not lead to a significant increase in prolactin in the blood plasma, but can cause weight gain and the development of edema due to hypersecretion of antidiuretic hormone. | |||
Acute dystonia
(or early dyskinesias) occur in 25–75% of cases in the first 7–10 days after starting therapy with classical antipsychotics or increasing the dose of previously taken drugs and are characterized by the sudden appearance of motor disorders of a spastic tetanoid nature.
Motor disturbances can be local and occur in typical areas, affecting an isolated muscle group, or generalized, accompanied by general motor agitation with the effects of fear, anxiety, narrowing of consciousness and autonomic disorders (profuse sweat, hypersalivation, lacrimation, vasomotor reactions, etc.). With local dystonias, tongue convulsions, trismus, hyperkinesis of facial muscles, gaze spasms (oculogyric crises), torticollis, opisthotonus, dyspnea, etc. occur. Oral syndrome (Kulenkampff-Tarnow) has also been described, which manifests itself as an unexpected tonic contraction of the muscles of the neck, mouth, protruding tongue , impaired phonation and breathing. In some cases, these symptoms can be regarded as manifestations of epilepsy or infectious diseases of the central nervous system (meningitis, encephalitis, etc.). Treatment.
With the development of local dystonia, the most effective is intramuscular or intravenous administration of akinetone in a dose of 5 mg (G.Ya. Avrutsky, D.I. Malin, 1994).
In the absence of the drug, dystonic reactions can be stopped with aminazine - 25–50 mg intramuscularly and 2 ml of a 20% caffeine solution subcutaneously (G.Ya. Avrutsky, I.Ya. Gurovich, V.V. Gromova, 1974). For generalized dystonia, simultaneous administration of aminazine or tizercin in a dose of up to 50 mg intramuscularly and antiparkinsonian correctors (akineton 5 mg intramuscularly) is indicated. Acute dystonia can be stopped by prescribing diazepam (Relanium) 20 mg intravenously slowly or intramuscularly. To prevent re-development of dyskinesias, antiparkinsonian correctors are prescribed or their dose is increased. Akathisia
occurs within the first 4 weeks after the start of antipsychotic therapy or an increase in the dose of antipsychotics and is characterized by the appearance of complaints of restlessness, restlessness, the need to move, and change body position.
Patients become fussy, trample from foot to foot, are forced to constantly walk in order to relieve anxiety, and cannot sit or stand still for several minutes. Akathisia can be combined with neuroleptic parkinsonism. Rare “late” cases of akathisia have also been described, in which there is no rapid response to the prescription of antiparkinsonian correctors and a reduction in the dose of antipsychotics. These cases are difficult to distinguish from tardive dyskinesia (M.Munetz, C.Cornes, 1982). Treatment.
Antiparkinsonian correctors cyclodol, akineton, etc. The administration of tranquilizers - diazepam, clonazepam, phenazepam in average therapeutic doses - is also effective.
Tardive dyskinesias
are one of the most severe neurological complications of neuroleptic therapy and develop in 20–30% of patients constantly taking classical antipsychotics.
The incidence of tardive dyskinesia in young people taking antipsychotic therapy for a year is 5%, in older people – 25–30%. When treated with atypical neuroleptics (Rispolept, Zyprexa), tardive dyskinesias develop much less frequently. Thus, according to research by P. Lemmens et al. (1999) during treatment with Rispolept for a year, tardive dyskinesias were noted in 0.23% of patients. According to DSM-IV, motor disturbances in tardive dyskinesias must persist for more than 4 weeks after discontinuation of antipsychotic therapy. They may occur during long-term use of antipsychotics or appear within the first 4 weeks after discontinuation of conventional antipsychotics and 8 weeks after discontinuation of long-acting antipsychotics. The clinical picture of this complication is characterized by the gradual development of various hyperkinesis (oral, athetoid, choreiform, torsion-dystonic) with a tendency to their generalization. In other cases, hyperkinesis may appear after sudden cessation of antipsychotic medications. Often hyperkinesis intensifies during breaks between courses of therapy, while other extrapyramidal disorders undergo reverse development. Simultaneously with neurological changes, persistent changes also occur in the mental sphere. Their combination is described as manifestations of psychopharmacotoxic encephalopathy (I.Ya. Gurovich, E.P. Fleiss, 1969). They are characterized by the passivity of patients, increased psychophysical exhaustion, affective instability, slowing of intellectual processes, importunity, as well as phenomena of “hysterization” of the psyche with a tendency to demonstrably intensify existing dyskinesias. Treatment.
When the first signs of the development of tardive dyskinesia appear, it is necessary to discontinue antipsychotics (provided that the patient’s mental state allows this). In cases where therapy cannot be stopped, preference should be given to atypical antipsychotics (azaleptin, rispolept, Zyprexa), in which the risk of developing complications is significantly lower. It has been established that in half of the cases tardive dyskinesia disappears after stopping the medication. Moreover, after discontinuation of neuroleptics, dyskinesias can worsen, and improvement in many cases occurs within several months (J. Kane, J. Smith, 1982). To reduce dyskinesias, the use of the antioxidant alpha-tocopherol (vitamin E) is effective. Due to the presence of cerebral organic insufficiency in many patients, the treatment regimen should include drugs with neurometabolic action (nootropil, picamilon, pantogam, phenibut, etc.), methods of restorative and physical therapy. Baclofen 15–30 mg per day and sodium valproate 400–600 mg per day are also recommended. If dyskinesias do not disappear, then patients are prescribed antipsychotics in low doses - Sonapax 50–150 mg/day, Leponex 50–100 mg/day. The most effective is the use of tiapridal 200–600 mg/day. It is also recommended to take benzodiazepines - diazepam 10-30 mg/day, clonazepam 2-6 mg/day. The use of antiparkinsonian correctors with central anticholinergic activity in chronic extrapyramidal neuroleptic syndrome is ineffective. Some reduction in the severity of dyskinesia can be achieved with the use of Akineton, which, in our opinion, has a more effective effect on hyperkinetic manifestations compared to other antiparkinsonian drugs. In addition, the presence of an ampoule form allows the use of akineton for parenteral - intramuscular and intravenous drip administration, which enhances the therapeutic effect (G.Ya. Avrutsky, D.I. Malin, 1994). Some authors note the possibility of increased dyskinesia when using anticholinergic correctors (T. Friis, J. Gerlach, 1983; W. Greil et al., 1984). Our studies have shown that anticholinergic correctors have a positive effect if, simultaneously with dyskinesia, parkinsonism phenomena are observed in the form of an amyostatic symptom complex with a plastic increase in muscle tone. It is assumed that the development of dyskinesia is associated with hypersensitivity of dopamine receptors. It is possible that autoimmune mechanisms may be involved in this process. Recently, it has been found that the autoimmune process can directly affect the structures of the dopamine system at the level of dopamine receptors with the formation of antireceptor antibodies with stimulating and blocking effects (G.I. Kolyaskina, T.P. Sekirina, 1990). From these positions, the use of extracorporeal detoxification methods that have a detoxifying and immunocorrective effect may be theoretically justified. The results of our own studies showed that after plasmapheresis and hemosorption, simultaneously with a decrease in motor disorders, an improvement in mental and general physical condition is observed - a decrease in lethargy, apathy, increased activity, improved sleep, and appetite. Thus, along with extrapyramidal symptoms, manifestations of the psychoorganic syndrome are also reduced.
Table 3. Treatment of extrapyramidal side effects of antipsychotics
| By-effect | Treatment |
| Parkinsonism | Prescription of anticholinergics |
| proofreaders: | |
| cyclodol 2-18 mg/s | |
| Akineton 2-24 mg/s | |
| tremblex 0.25-0.5 (2-4 ml) | |
| intramuscularly | |
| benzotropine 3-9 mg/s | |
| Acute dystonia | Akineton 5-10 mg/s intramuscularly, |
| intravenously | |
| Relanium 10-20 mg intramuscularly, | |
| intravenously | |
| In the absence of akineton, chlorpromazine | |
| 25-50 mg intramuscularly + 2 ml | |
| 20% caffeine solution subcutaneously | |
| For the purpose of prevention, increase | |
| daily dose of correctors | |
| Akathisia | Anticholinergic correctors |
| Tranquilizers - diazepam, clonazepam, | |
| phenazepam in average therapeutic doses. | |
| Tardive dyskinesia | Low doses of some antipsychotics: |
| Sonapax 50-150 mg/s; leponex 50-100 mg/s | |
| The most effective is tiapride 200-600 mg/s | |
| Tranquilizers - clonazepam 2-6 mg/s; | |
| diazepam 20-30 mg/s | |
| Akineton is effective in some cases | |
| Vitamin E | |
| Nootropics | |
| Extracorporeal detoxification methods | |
| (plasmapheresis, hemosorption) | |
| Neuroleptic malignant syndrome | Cancellation of antipsychotics |
| Prescription of intensive infusion | |
| therapy (from 2.5 to 6 l per day) | |
| Nootropics | |
| Bromocriptine 7.5-15 mg/s | |
| Dantrolene 100 mg/s | |
| Plasmapheresis, hemosorption |
Prevention of complications should be based on taking into account risk factors. It has been established that tardive dyskinesia occurs most often with the following predisposing factors: 1) the presence of cerebral organic insufficiency; 2) old age; 3) duration of use of high doses of antipsychotics, especially piperazine derivatives of phenothiazine and butyrophenones; 4) a tendency to develop massive extrapyramidal symptoms with a predominance of prolonged hyperkinesis. In the presence of these factors, especially when they are combined, therapy should be carried out with extreme caution, taking into account the possibility of complications (G.Ya. Avrutsky, I.Ya. Gurovich, V.V. Gromova, 1974).
2. Causes of the disease
The cause of these disorders is associated with damage to the extrapyramidal system of the brain and neurotransmitter imbalance. The extrapyramidal part of the brain ensures control of posture, smoothness of movements, and their compliance with the intended action. Accuracy, speed and coordination of different muscle groups are also controlled by this system.
Quite often, extrapyramidal disorders occur as a side effect of taking antipsychotic drugs.
. Drug-induced extrapyramidal disorders can also be observed when taking antidepressants, calcium antagonists, antiarrhythmic drugs and drugs prescribed for Parkinson's disease. Side effects of these drugs can occur in the first days of treatment or as a consequence of prolonged regular use (respectively, “early” and “late” drug disorders). Late extrapyramidal disorders can develop even after discontinuation of the drug and be irreversible. This risk must be taken into account when including these drugs in a therapeutic regimen.
Extrapyramidal disorders significantly reduce the quality of life of patients, severely limiting social activity. The psychological status is characterized by anxiety, feelings of inferiority, cognitive impairment, isolation, loss of interest in the outside world and serious feelings of loneliness.
Visit our Neurology page
Parkinsonism and its secondary forms: similarities and differences
Parkinsonism - a syndrome of movement disorders - in 70% of cases is a manifestation of Parkinson's disease, which is associated with natural aging of the body and/or hereditary predisposition, usually diagnosed in people over 60 years of age.
The remaining 30% are secondary forms of parkinsonism, which can develop at any age as a result of various damage to the central nervous system. Such pathologies can be classified according to the most common causes of their occurrence:
- drug-induced parkinsonism is a side effect of certain medications - antipsychotics, sympatholytics, antiepileptic drugs, etc.;
- toxic - usually equated to the dosage form, but with this type the leading role is played by poisoning with salts of heavy metals, salts of hydrocyanic acid, carbon monoxide, alcohol abuse, the use of surrogate and synthetic drugs, “ecstasy”;
- vascular - develops after a stroke, as well as against the background of other vascular pathologies (dyscirculatory encephalopathy, cerebral atherosclerosis, etc.);
- post-infectious - often regarded as the chronic stage of encephalitis, because it usually occurs against the background of this disease;
- hydrocephalic - a consequence of expansion of the ventricles of the brain without increasing intracranial pressure;
- post-traumatic - occurs after a serious traumatic brain injury or as a result of frequent head injuries of moderate and mild severity (among athletes, law enforcement officers).
The symptoms of Parkinson's disease and secondary forms of parkinsonism are approximately the same:
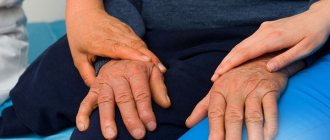
- tremor - trembling in the hands;
- hypokinesia - inactivity;
- muscle rigidity - constantly increased tone of the flexor muscles;
- impaired balance, coordination of movements, unsteady gait, etc.
Parkinsonism differs from Parkinson's disease in the following ways:
- faster onset of the disease;
- more malignant course, quickly leading to disability;
- less pronounced or complete absence of effect from antiparkinsonian treatment.
3.Types of extrapyramidal disorders
Ametosis
This type of disorder most often manifests itself in the hands and facial muscles. Characterized by slow wriggling movements of the fingers, which look worm-like and devoid of bones. Twitching of the lips and tongue, curvature and asymmetry may be observed on the face. The facial muscles alternately tense and relax. Such disorders can be the result of birth trauma, encephalitis, syphilis and traumatic brain injuries.
Chorea
This type of disorder is manifested by erratic, irregular movements of the entire body. At the same time, the muscles of the trunk and limbs are characterized by a decrease in tone.
Torsion spasm
A combination of dystonia of the trunk muscles with spasms, up to complete freezing of the entire body. This type of disorder begins with the neck muscles, which involuntarily turn the head to the side. This type of torsion torticollis can develop, involving other muscle groups. In some cases, a “writer’s cramp” is observed - while writing or even when trying to give the fingers a “writing” position, a spasm of the hand occurs due to hypertonicity in the fingers.
Teak
Involuntary repeated contractions of certain muscles (usually the face or neck). This disorder can range from eyelid twitching to obsessive winking, head tilting, and shoulder twitching. As a rule, stressful situations and anxiety intensify the manifestation of this type of extrapyramidal disorder.
Hemiballism
Sweeping unilateral movements of the limbs are observed, reminiscent of throwing up or an attempt to make a grasping movement. This type of obsessive movements most often develops against the background of an infectious brain lesion (tuberculosis, syphilis, encephalitis). It can also occur with severe vascular disorders and metastasis to the brain.
Tremor
Hand tremors, head tremor. When trying to make a precise movement, the amplitude and frequency of movements increase with increasing concentration on the object. In some forms (Parkinson's disease), a “rest tremor” is observed - trembling occurs in a static position, but does not appear during movement.
Facial hemispasm
Spasm of half the face, including the tongue, eyes and neck. This type may be accompanied by sounds such as laughter, crying, or screaming.
The listed types of extrapyramidal disorders are most often combined with each other in different combinations
and are included in the symptom complex of serious diseases of hereditary or acquired origin.
Severe metabolic and cerebral circulation disorders, trauma, and neuroinfections lead to muscle spasms and dystonia. Any changes in tone and loss of control over movements can be a manifestation of severe brain disorders and require immediate contact with a neurologist.
About our clinic Chistye Prudy metro station Medintercom page!
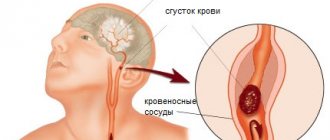
Extrapyramidal disorders in cerebrovascular diseases
The article discusses the most common post-stroke extrapyramidal disorders. Using the example of the drug Ceraxon®, the possibilities of neuroprotective therapy in the treatment of post-stroke extrapyramidal disorders are discussed.
Cerebrovascular diseases are the most important medical and social problem. The prevalence of cerebral stroke remains high throughout the world: 16.9 million strokes were registered in 2015, with an average incidence of 258 cases per 100 thousand population [1]. Every year, 5.9 million people die from stroke worldwide. The incidence of stroke in Russia is significantly higher than in Western European countries, amounting to 300–350 cases per 100 thousand population. In Moscow alone in 2014, over 35 thousand strokes occurred. The increase in the number of strokes among young people is also alarming. Extrapyramidal symptoms, which are characterized by great diversity, involvement of various structures in the pathological process, heterogeneity of treatment approaches and prognosis, are not among the frequent manifestations of acute stroke. However, the resulting disorders can be persistent and cause significant disability. Meanwhile, doctors are much less aware of post-stroke extrapyramidal disorders than of other focal symptoms.
Extrapyramidal disorders: epidemiology and pathogenesis
According to epidemiological data, extrapyramidal disorders are observed in 1–4% of patients who have had a stroke, in approximately equal proportions in men and women [2]. It is possible that the prevalence of post-stroke extrapyramidal disorders is greatly underestimated, since they can develop delayed (after several years), which makes it difficult to take them into account in epidemiological studies.
The main role of the basal ganglia in the system of organizing voluntary movements is to regulate the motor activity of the cortex, prepare for movement, regulate muscle tone and control the sequence of activation of various muscle groups. A kind of “exit gate” of the basal ganglia is the internal segment of the globus pallidus and the reticular part of the substantia nigra. The total vector of activity of the direct and indirect dopaminergic pathways is realized on these structures. Normally, inhibition of the globus pallidus and the reticular part of the substantia nigra occurs, and thalamocortical projections are disinhibited, which leads to facilitation of the movement initiated by the cortex. At the same time, the cortex, through direct corticostriatal and corticosubthalamic pathways, has a regulatory effect on the basal ganglia. In addition, there are numerous activating and inhibitory connections between individual structures of the basal ganglia. In a simplified way, the development of hypokinetic syndrome can be explained by increased activity of the indirect pathway, hyperkinetic disorders - by the direct pathway.
Despite the fact that the basal ganglia play a huge role in the control of voluntary movements, severe damage to the basal ganglia due to stroke is not always accompanied by motor impairment. According to the Lausanne Stroke Registry, out of 2500 patients, only 29 (1%) had post-stroke extrapyramidal disorders [3]. Today, the question of why morphological damage to the basal ganglia in some cases is not accompanied by clinical symptoms remains open. Probably, such factors as individual sensitivity to ischemia of neuronal subcortical structures, the possibility of plasticity of brain tissue, and the inclusion of compensatory mechanisms have an influence. In addition, apparently, for the disruption of the basal ganglia, it is not so much a single, lacunar lesion that is important, but rather the dysfunction of a number of interneuronal connections [4, 5]. Indeed, in the mechanisms of development of akinetic-rigid syndrome in Parkinson's disease, not only dysfunction of the direct and indirect dopaminergic pathways due to disruption of dopamine production by neurons of the substantia nigra plays a role, but also changes in the functional activity of cortical and stem structures. Thus, according to positron emission tomography with fluorodeoxyglucose, there is a decrease in the activity of metabolic processes in the premotor and supplementary motor cortex, as well as in the associative zones of the temporal lobe [6].
Post-stroke extrapyramidal disorders
Post-stroke extrapyramidal disorders are represented, as a rule, by unilateral disorders contralateral to the stroke focus (83% of cases), but bilateral symptoms are also possible [3]. In addition, stroke in the brainstem or cerebellum may result in unilateral ipsilateral symptoms.
The most common extrapyramidal syndromes associated with stroke include hemichorea (with or without hemiballismus), dystonia, tremor, parkinsonism, or myoclonus [3, 7]. Post-stroke extrapyramidal disorders can develop acutely, along with other focal manifestations of stroke, and delayed (after several weeks, months and even years), and also progress over time [2]. Options for the transformation of hyperkinesis from hemiballismus in the acute period to hemichorea and later to hemidystonia have been described [8].
There is a relationship between the timing of the formation of extrapyramidal symptoms and their nature [9–11]. Thus, chorea appears much earlier than the symptoms of parkinsonism. In a study by F. Alarcón et al., the average time to develop hemichorea was 4.3 days, parkinsonism was 117.5 days (p
A correlation was found between the time of development of hyperkinesis, its nature and the age of the patients. BL Scott and J. Jankovic found that at a young age, extrapyramidal symptoms do not appear immediately [11]. Thus, in two patients (average age 28.7 years) with ischemic infarctions, hemidystonia on the side of hemiparesis debuted after 42.8 years. In elderly patients, the latent period for the development of symptoms was one to four years. In addition, young patients are characterized by a tendency toward generalized hyperkinesis, while older patients have focal or segmental forms of dystonia [11].
According to F. Alarcón et al., dystonia is more common in young people with stroke, and chorea is more common in older age groups [7].
The pathophysiology of delayed onset of extrapyramidal disorders is not entirely clear. This phenomenon is characteristic not only of stroke, but also of traumatic brain injury. The hypothesis that explains it is the role of synaptic plasticity, which leads to the gradual formation of new functional connections in the system of subcortical-cortical circles, changes in the balance of activating and inhibitory influences and, as a result, the formation of abnormal motor patterns [2].
Extrapyramidal symptoms are most often caused by focal changes in the striatum/pallidum (44%) and thalamus [3]. There is no clear correlation between the localization of the stroke focus and the nature of hyperkinesis. Moreover, damage to the same formations can lead to different extrapyramidal symptoms. Thus, the cause of hemiballismus can be damage not only to the subthalamic nucleus, but also to the striatum and thalamus. Hemidystonia, hemichorea, and hemiathetosis occur due to damage to both the lentiform and caudate nuclei [3]. After a stroke, chorea, athetosis, or dystonia may develop in the thalamus. Extrapyramidal syndromes most often occur due to small deep infarctions against the background of microangiopathy [3, 7, 11]. However, cases of hyperkinesis have been described after cardioembolic or atherothrombotic stroke, as well as after parenchymal or subarachnoid hemorrhage [4].
Vascular chorea
Chorea is the most common post-stroke hyperkinesis. It is observed in 0.4–1.3% of patients who have suffered acute cerebrovascular accident, mainly in elderly people [11]. Choreic hyperkinesis debuts acutely in the first four days after the stroke [3, 11]. Hyperkinesis is most often represented by hemichorea or in the case of bilateral vascular lesions it can be generalized. In most patients it is accompanied by muscle weakness on the same side. Cases without paresis are less common. In addition, several cases of simultaneous presence of contralateral hemichorea and hemiparesis on the opposite side have been described [3, 13]. Choreic hyperkinesis, despite the restoration of muscle strength in the limbs, can persist and become chronic. In severe cases, chorea can be combined with throwing movements, that is, it can develop into hemiballismus. The latter, unlike chorea, involves not only a large range of movements, but also the mandatory involvement of the proximal limbs.
Vascular chorea develops as a result of ischemic or hemorrhagic damage to the thalamus, lentiform nuclei, and less commonly, the subthalamic nucleus (the area of blood supply to the lateral lenticulostriate or thalamoperforating arteries - the basin of the middle and posterior cerebral arteries) [12, 13]. Several studies have shown that vascular chorea can be caused by damage to the frontal, temporal or parietal lobes. Hyperkinesis in this case is the result of a decrease in the activating influence of the cortex on the subcortical ganglia and the functional inactivation of the latter. Another explanation is the presence of small lesions in the basal ganglia not identified by structural neuroimaging. Thus, N. Mizushima et al. described a patient with a right temporal lobe infarction and contralateral hemichorea who showed reduced perfusion in the right basal ganglia only using single-photon emission computed tomography [14]. A case of hemichorea was also observed in stenosis of the extracranial part of the internal carotid artery, with hypoperfusion in the small arteries of the basal ganglia, detected by functional neuroimaging, and a reduction in hyperkinesis after revascularization.
The development of hemiballismus is associated with predominant damage to the subthalamic nucleus (according to clinicopathological studies, damage to at least 20% of the substance of the subthalamic nucleus). Hemiballismus may be a consequence of a hemorrhagic focus in the striatum.
Spontaneous regression of choreic hyperkinesis against the background of acute stroke is observed in 50% of cases. In some patients, hyperkinesis may be persistent. Observations show that the prognosis is better in patients with cortical strokes compared to those who suffered subcortical infarctions. This is consistent with the assumption that chorea in cortical strokes is a consequence of transient hypoperfusion of the subcortical-thalamic pathways or their functional inactivation. In the case of hemiballismus, the same pattern is observed - the prognosis is better in the case of a cortical lesion.
The management strategy for patients with vascular chorea is the same as for patients with acute stroke - vasoactive, metabolic, antiplatelet therapy. However, if hyperkinesis becomes pronounced and is accompanied by a large range of movements (chorea and hemiballismus), it is necessary to prescribe symptomatic therapy aimed at blocking dopamine receptors. For treatment, typical antipsychotics (haloperidol, sulpiride, pimozide) are used in low doses. The duration of their use should be limited to two weeks with gradual withdrawal [15]. The effectiveness of atypical antipsychotics, such as risperidone, quetiapine, olanzapine, clozapine, has been confirmed in a number of studies [16]. Good results have been observed with tetrabenazine, an atypical antipsychotic that depletes the pool of presynaptic dopamine and is widely used throughout the world for the treatment of Huntington's chorea.
In addition to neuroleptics, benzodiazepines (clonazepam, diazepam), valproic acid preparations, gabapentin, trihexyphenidyl are prescribed as symptomatic treatment for vascular chorea. There are isolated reports of the effectiveness of the use of amantadines. Several randomized placebo-controlled studies showed that intravenous administration of 400 mg/day amantadine led to a significant reduction in the severity of hyperkinesis [17].
Surgical treatment of vascular chorea is not widespread. However, in a number of severe cases of hemichorea/ballisma (lasting more than one year), stereotactic operations on the thalamus or posteroventral pallidotomy had a positive effect [18]. Deep brain stimulation (thalamus, internal segment of the globus pallidus) is currently also considered as a way to correct choreic hyperkinesis. Some studies have demonstrated at least a three-year improvement in well-being [19].
Dystonia
Dystonia is the second most common (after chorea) hyperkinesis. Occurs several weeks or months after the stroke (on average nine months). Post-stroke dystonia is formed contralateral to the lesion located in the putamen or thalamus, less often in the globus pallidus [2, 4]. Cases of isolated hand dystonia as a result of a stroke in the parietal lobe have been described.
Dystonia is most often focal, but can be segmental or generalized (hemidystonia). Post-stroke hemidystonia in childhood is often combined with hemiatrophy [8]. Typically, the development of a dystonic position of the hand with flexion in the area of the metacarpophalangeal joints and extension of the interphalangeal joints, adduction and flexion of the elbow. With muscle dystonia in the leg, an equinovarus position of the foot is formed with or without an extension position of the big toe (similar to Babinsky’s symptom). Cases have been described where dystonia of the hand is joined after a few months by dystonia of the foot [8]. Cranial dystonia (blepharospasm, oromandibular dystonia) is extremely rare (damage to the brainstem-diencephalic projections or thalamus) [21]. Cervical dystonia may be a consequence of an ischemic or hemorrhagic lesion in the cerebellar hemisphere, pons, or tegmentum [22].
Treatment of post-stroke dystonia requires an integrated approach with the prescription of anticholinergics, clonazepam, carbamazepine, valproate, and a baclofen pump. For focal dystonia, botulinum toxin injections are used. In severe cases, surgical intervention is recommended - destruction of the globus pallidus or thalamic nuclei, as well as deep brain stimulation.
Vascular parkinsonism
The concept of vascular parkinsonism was first put forward in 1929 by M. Critchley [23]. He described the clinical manifestations of parkinsonism that developed against the background of cerebrovascular disease in elderly patients and proposed the term “atherosclerotic parkinsonism.” He listed rigidity, a mask-like face, and a shuffling gait as the main symptoms of atherosclerotic parkinsonism. Depending on additional symptoms (pseudobulbar palsy, dementia and urinary incontinence, pyramidal symptoms, cerebellar insufficiency), M. Critchley identified six types of the disease [23]. M. Critchley's concept came under serious criticism in subsequent years, since clinically Parkinson's disease and parkinsonism due to cerebrovascular disease are difficult to differentiate. Many patients with Parkinson's disease have vascular risk factors. Moreover, the presence of a vascular component aggravates the course of the disease. Subsequently accumulated pathological data confirmed the correctness of M. Critchley's assumption about the probable vascular nature of the disease. However, even today, despite the capabilities of neuroimaging, problems often arise in making a diagnosis.
According to epidemiological data, vascular parkinsonism accounts for 3–12% of all cases of parkinsonism [24]. The prevalence of vascular parkinsonism, according to pathologically confirmed studies, ranges from 1–6% [24]. Thus, in the work of KA Jellenger, during an autopsy of 759 patients with parkinsonism, vascular etiology was confirmed in 3.4% of cases [25].
Vascular parkinsonism most often develops against the background of multiple lesions of the basal ganglia. Less commonly, parkinsonism results from single infarcts in the thalamus, lenticular nuclei, or pons. It should be emphasized that only a very small number of patients with vascular pathology and even those with vascular lesions show symptoms of parkinsonism [24]. I. Reider-Groswasser found that only 38% of patients with focal changes in the basal ganglia suffered from parkinsonism [26].
Lacunar infarctions are often combined with leukoaraiosis and damage to the white matter of the frontal lobes. In a study of 14 patients with vascular parkinsonism, the most characteristic neuroimaging finding was a combination of lacunar changes in the striatum and leukoaraiosis [2]. It is extremely rare that a focal lesion affects the substantia nigra, but in these cases the clinical picture of the disease is identical to Parkinson's disease.
The basis of lacunar lesions of the basal ganglia in vascular parkinsonism is most often microangiopathy associated with hypertension or diabetes mellitus [27]. Cases of vascular parkinsonism have been described in CADASIL syndrome and Moyamoya disease [2]. Another cause of lacunar changes in the basal ganglia is lipohyalinosis of small vessels, leading to microocclusion, ischemia and gliosis. Pathomorphological examination reveals proliferation of astrocytes, demyelination of axons, and expansion of perivascular spaces. As a result, multiple lacunar changes are formed in the basal ganglia, internal capsule, and center semiovale [24].
Based on the nature of development, two forms of vascular parkinsonism are distinguished:
- with an acute onset due to extensive lacunar infarction in the basal ganglia and stepwise progression of symptoms;
- gradual onset and slow progression against the background of diffuse damage to the subcortical white matter in combination with ischemic changes in the striatum, lenticular nuclei or pons. A similar variant of the course of vascular parkinsonism occurs in every second or third patient with vascular parkinsonism.
Classic variants of vascular parkinsonism include cases of parkinsonism of the “lower body” - with bilateral symmetrical symptoms in the form of bradykinesia, rigidity in the legs and gait disturbances (decreased length and height of step, wide base, shuffling, postural instability, tendency to fall). Often in patients with vascular parkinsonism there is a starting delay when walking and a tendency to propulsion. Unlike Parkinson's disease, acheirokinesis (lack of cooperative arm movements when walking) is not typical for vascular parkinsonism. The presence of a resting tremor of the “pill rolling” type with a frequency of 4–6 Hz excludes the diagnosis of vascular parkinsonism. Slight postural or kinetic tremor may occur. Rigidity of the “cogwheel” type is observed very rarely. A combination of rigidity and spasticity is more common, predominantly in the lower extremities. In almost all patients (80%) with vascular parkinsonism, pyramidal symptoms (increased tendon and periosteal reflexes, Babinski's symptom), cognitive impairment, urinary disorders, and symptoms of pseudobulbar palsy can be detected. However, there are cases of vascular parkinsonism with a less obvious picture. Thus, in an Italian multicenter cross-sectional study in patients with diagnosed vascular parkinsonism, an asymmetric onset occurred in 59% of cases [2]. Hemiparkinsonism usually develops in the first months after an acute cerebrovascular accident and is associated with a large lesion in the basal ganglia.
The diagnosis of vascular parkinsonism must be confirmed by neuroimaging data in the form of focal changes in the basal ganglia, thalamus, and frontal lobe. In most cases, the DAT scan does not reveal any disturbances in binding to the dopamine transporter. At the same time, in vascular parkinsonism, changes, according to functional neuroimaging, can occur, but, unlike Parkinson’s disease, they are symmetrical in nature [24]. An additional diagnostic test may be a test of olfactory function. Anosmia is characteristic of Parkinson's disease and diffuse Lewy body disease, but not of vascular parkinsonism.
Dopaminergic therapy is ineffective for vascular parkinsonism. Only 20–30% of patients experience a moderate short-term effect from taking levodopa, and, as a rule, high doses of levodopa are used – 750–1500 mg/day [28]. If positive dynamics are not achieved within four to six weeks, then there is no point in continuing treatment with levodopa. A number of studies have noted that patients with changes on the DAT scan respond better to levodopa [28]. To improve the walking pattern, it is recommended to use rhythmic sound signals (metronome, music), visual references in the form of transverse stripes drawn on the floor corresponding to the width of the step, and a cane with a transverse crossbar. Courses of vasoactive and metabolic therapy are of particular importance in accordance with the principles of treatment of chronic cerebrovascular insufficiency and post-stroke conditions.
Tremor
Isolated post-stroke tremor is extremely rare. Typically, this is a unilateral postural or kinetic tremor. Tremor is one of the delayed post-stroke hyperkinesis. Tremor does not occur as a symptom of the acute period of stroke [29]. The development of hyperkinesis is caused by damage to the thalamus or dentato-rubro-thalamic, cerebellar-thalamic or nigrostriatal pathways. Cases of isolated tremor when writing as a result of lacunar infarction of the frontal lobe have been described. Damage to the midbrain is associated with Holmes tremor - unilateral, predominantly involving the proximal parts of the limbs, occurring at rest, while maintaining a pose (postural) and intensifying with movement [30]. Post-stroke tremor is practically refractory to pharmacotherapy. Propranolol or primidone are rarely effective. In severe cases, deep brain stimulation with implantation of electrodes into the ventral nuclei of the thalamus is recommended [24].
Myoclonus
Myoclonus rarely develops after an acute stroke. There are no reports in the literature of generalized myoclonus manifested in the late period of acute cerebrovascular accident. There are isolated descriptions of asterixis (negative myoclonus) - a repeated rhythmic drop in muscle tone in the hands when trying to hold them in a horizontal position. Asterixis in these cases was unilateral (contralateral to the lesion) or bilateral in nature. The appearance of asterixis is associated with damage to the thalamus, possibly in combination with the subthalamic nucleus [31, 32].
The role of neuroprotective therapy in post-stroke extrapyramidal disorders
The treatment of extrapyramidal disorders as focal symptoms that develop in the acute period of stroke is based on the principles of pathogenetic therapy of ischemic or hemorrhagic stroke. One of the areas of therapy in the acute period is the use of neuroprotective drugs. Among them, preference should be given to drugs with a convincing evidence base, with a multimodal effect and a proven safety profile.
Citicoline (Ceraxon®) is widely used in clinical practice for both ischemic and hemorrhagic stroke. Administration of citicoline for stroke significantly reduces mortality rates and permanent disability. A number of studies using computed tomography and magnetic resonance imaging have shown that the administration of citicoline on the first day of ischemic stroke led to a decrease in the volume of the ischemic lesion. The effect of the drug was dose-dependent (dosages of 500, 1000, 2000 mg/day were used). The average increase in lesion volume during therapy with Ceraxon® at a dose of 2000 mg/day was only 1.8% [33]. A. Davalos et al. Four clinical trials examined the results of oral citicoline in 1372 patients with ischemic stroke [34]. Citicoline was prescribed starting from the first day of the disease for six weeks. By week 12, recovery was achieved in 25% of cases in the citicoline group and 20% in the placebo group (p
All clinical studies emphasize the good tolerability and safety of the drug [33–35]. Citicoline is included in European recommendations and Russian standards for the treatment of stroke [34, 36].
The neuroprotective properties of the drug are associated with a pronounced membrane-stabilizing effect. Citicoline is involved in the synthesis of the main phospholipids (phosphatidylcholine, sphingomyelin, cardiolipin) of cell membranes [37–40]. By providing a direct repair effect, citicoline prevents damage to the cell surface and mitochondrial membranes when exposed to ischemia/hypoxia factors, as well as in neurodegenerative diseases. By stabilizing membranes, citicoline prevents the breakdown of phospholipids into fatty acids and the formation of free radicals [37–40]. The additional protective effect may be explained by an increase in the expression in brain neurons of the most important factor of endogenous neuroprotection, the protein sirtuin 1. Citicoline has a multicomponent neurotransmitter effect, promoting the synthesis of acetylcholine, serotonin, and norepinephrine [41]. The ability of citicoline to influence glutamatergic and GABA receptors has been noted [41]. In a number of experimental models of parkinsonism, the drug increased the level of dopamine in the striatum, stimulating its release by increasing the activity of tyrosine hydroxylase [42, 43]. Another reason for increased dopamine levels is inhibition of dopamine reuptake, possibly related to the effect of citicoline on phospholipid synthesis [44].
The dopamine-stimulating effect of citicoline served as the basis for its inclusion in the complex therapy of Parkinson's disease. Several double-blind crossover studies have demonstrated that citicoline administered as an intravenous infusion at a dose of 500 mg/day for 10–20 days improved motor performance and reduced bradykinesia, rigidity, and tremor. Improvement was observed when citicoline was prescribed both in monotherapy and in combination with levodopa [45, 46].
In the study by J. Acosta, citicoline was prescribed at a dose of 500 mg/day (with a stable dose of levodopa) for ten days intravenously, and then for 14 days as an oral solution [47]. At the end of the course of therapy, 36% of patients showed a good effect, mainly in relation to bradykinesia and rigidity. No significant effect on tremor was noted. According to an analysis of effectiveness depending on the time of administration of citicoline, the best results were observed in patients who were on levodopa therapy for less than two years. In some patients, the addition of citicoline reduced the dose of levodopa by 25–30% [47]. These data were confirmed by R. Eberhardt et al., who concluded that the administration of citicoline can reduce the dose of levodopa and thereby reduce the risk of adverse reactions associated with its use [48].
In a multicenter, blind, placebo-controlled study, C. Loeb et al. During the administration of citicoline at a dose of 1000 mg/day intravenously, in addition to the ongoing therapy, not only a significant positive effect of the drug was noted in comparison with placebo, but also a deterioration in the condition of patients after discontinuation of citicoline. This demonstrates the effectiveness of citicoline as an adjuvant agent during levodopa therapy in patients with Parkinson's disease [49].
Taking into account the neuroprotective effect and neurotransmitter potential, Ceraxon® is also indicated in the acute period of stroke, accompanied by extrapyramidal symptoms. Ceraxon® may be effective in the complex treatment of vascular parkinsonism. Experimental studies have proven the trophic and neuroprotective effects of citicoline on nigrostriatal dopaminergic neurons. In particular, citicoline protects dopaminergic neurons from the toxic effects of methyl 4-phenylpyridine [50, 51] and glutamate [50]. The recommended doses of the drug Ceraxon® in the acute period of stroke are 1000 mg/day intravenously every 12 hours, starting from the first day, followed by switching to oral forms (packaged form of the drug with a drinking solution). It is important to emphasize that intravenous and oral forms of the drug Ceraxon® have the same bioavailability. Treatment with Ceraxon® should continue for at least six weeks.