Diagnostics
To determine multiple sclerosis, doctors use interviews and neurological examinations, and then confirm the diagnosis with MRI studies and tests.
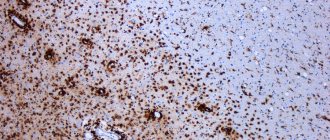
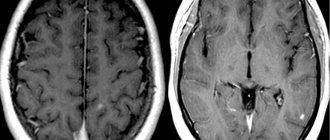
MRI scans the brain and spinal cord for old and new lesions. For better visualization of fresh plaques, contrast is used. The diagnosis is made if the patient has 4 or more foci of demyelination measuring at least 3 mm, or 3 foci near the lateral ventricles, in the brain stem, cerebellum or spinal cord. In addition to MRI, the following may be prescribed for multiple sclerosis:
- regular blood tests to determine the level of immunoglobulins in the cerebrospinal fluid;
- lumbar punctures to study the composition of the spinal cord fluid.
Symptomatic treatment
The goal of symptomatic therapy is to eliminate or alleviate existing neurological symptoms. Symptomatic therapy has no effect on the course of MS itself, but can improve the patient’s quality of life. For this purpose, drugs of various groups are used. Often, the same remedy simultaneously eliminates several manifestations of multiple sclerosis.
Synthetic analogues of GABA
Gamma-aminobutyric acid is one of the so-called. inhibitory mediators in the central nervous system. Damage to brain structures in multiple sclerosis is accompanied by the elimination of inhibitory impulses with the development of muscle spasms and spastic pain. Such symptoms are eliminated with the help of Baclofen. This synthetic analogue of GABA restores inhibitory impulses and inhibits spasm. Baclofen relaxes not only skeletal but also smooth muscles. Therefore, it can be used for spastic constipation and difficulty emptying the bladder.
Benzodiazepines
Benzodiazepine derivatives (Diazepam, Clonazepam) are similar in properties to GABA. They enhance the effect of Baclofen and also have tranquilizer properties. Therefore, they are used to eliminate anxiety and fear in patients with multiple sclerosis. In addition, benzodiazepines have analgesic properties - they can eliminate pain.
Anticholinergics
These drugs normalize muscle tone by influencing central and peripheral cholinergic receptors. "Mydocalm", "Hyoscyamine", "Oxybutynin" are used to relieve spasms of skeletal muscles, smooth muscles of the intestines and bladder.
Antiepileptic drugs
They can also be useful for multiple sclerosis. “Carbamazepine”, “Phenytoin” prevent the development of seizures, normalize sensitivity, and are effective for neuritis accompanied by pain, impaired swallowing and speech.
Tricyclic antidepressants
Eliminates feelings of depression and fatigue, improves mood and performance. These drugs also normalize muscle tone and eliminate pain.
Vitamins
The importance of these biologically active substances in multiple sclerosis is difficult to overestimate. B vitamins normalize sensitivity and movement, ascorbic acid improves immunity, vitamin E strengthens blood vessels, and vitamin D prevents the development of osteoporosis, a frequent companion of multiple sclerosis. All drugs for the symptomatic treatment of multiple sclerosis are used only as prescribed by a doctor and under his supervision.
Causes of multiple sclerosis
Modern medicine cannot give an exact answer to this question. The generally accepted opinion of doctors is the presence of unfavorable external and internal factors affecting the patient. Such unfavorable factors include:
- Poor nutrition
- Frequent viral or bacterial infections
- Spinal and brain injuries
- Stressful situations
- Genetic predisposition
- Radiation and toxic substances
- Geoeconomic place of growing up and living
- Vitamin D deficiency
- Autoimmune reactions in the body
Treatment of exacerbations
During exacerbations, therapy is carried out using potent anti-inflammatory drugs and corticosteroid hormones. The regimens and doses of administration of these drugs are individually determined by the doctor depending on the patient’s condition. Along with corticosteroids, during an exacerbation, drugs that improve the functions of nervous tissue (so-called neuroprotectors), drugs that strengthen the vascular wall, and drugs that improve the rheological properties of the blood are usually prescribed. In the absence of contraindications, physical therapy and physiotherapeutic procedures are indicated.
Pathogenetic treatment
The goal of pathogenetic treatment of MS is to quickly relieve symptoms caused by exacerbations, as well as to prevent exacerbations and increase the period of remission. In addition, one should strive to change the course of the disease and slow its progression. Medicines used to treat MS can be divided into two groups. The first group is drugs that are used during periods of exacerbation. The second group is drugs that change the course of the disease by suppressing the pathologically active immune system. These include immunomodulators, immunosuppressants, cytostatics and monoclonal antibodies. Of course, the most effective type of treatment for multiple sclerosis would be etiological, aimed at eliminating the cause. But since the etiology of the disease is still poorly understood, treatment begins with influencing the pathogenesis, the mechanism of development of the disease.
Pathogenetic treatment is aimed at:
- preventing axonal demyelination;
- acceleration of remyelination;
- immunosuppression - a decrease in the pathological activity of the immune system;
- immunomodulation – change, modulation of the immune response;
- inhibition of local inflammatory and autoimmune processes;
- strengthening, reducing the permeability of the vascular wall and the BBB (blood-brain barrier).
With properly selected pathogenetic treatment, recovery processes begin to prevail over the processes of damage to the axons of the myelin sheath of nerve fibers. As a result, the severity of exacerbations is reduced, and remissions, on the contrary, are lengthened. In this case, the transition of a relapsing course into a secondary progressive course with disability occurs as late as possible or does not occur at all.
Multiple sclerosis (MS) is a chronic progressive disease of the nervous system of unknown etiology, characterized by the development of diffuse demyelination and axonal degeneration [1]. It is classified as a multifactorial disease. This term refers to the combined effects of external and genetic factors, leading to the development of chronic inflammatory, demyelinating and neurodegenerative processes. Among external factors, special attention is currently paid to three risk factors for the development of MS: vitamin D deficiency, Epstein-Barr viral infection and smoking [2, 3]. Vitamin D deficiency has long been discussed as a risk factor for MS, but interest in it has increased dramatically in recent years.
The name “vitamin D” is given to a group of substances represented by more than 10 structural analogues. The main ones are vitamin D2, or ergocalciferol, and vitamin D3, or cholecalciferol. Vitamin D enters the body in two ways: with food and as a result of synthesis in the skin under the influence of ultraviolet rays. Absorption of vitamin D from food occurs in the duodenum and jejunum in the presence of bile acids. In the skin, under the influence of sunlight or ultraviolet radiation, previtamin D3 is formed from 7-dehydrocholesterol, which is immediately converted into vitamin D3 (cholecalciferol) under the influence of heat. Vitamin D formed in the skin and received from the intestines binds to a specific vitamin D-binding protein, which transports it to places of further metabolism. Part of vitamin D is transported to fat and muscle tissue, where it is fixed, representing a reserve form, but the main amount is transferred to the liver. In the liver, with the help of the enzyme 25-hydroxylase, vitamins D2 and D3 are converted into 25-hydroxyvitamin D [25(OH) D3], or calcidiol, the main circulating form of vitamin D. Then in the kidneys, calcidiol is converted into the active metabolite 1 by the enzyme α-hydroxylase ,25-dihydroxyvitamin D3 (calcitriol). This metabolite is recognized by tissues containing specific vitamin D receptors (VDRs), which are present in the skin, bones, muscles, gonads, intestines, central nervous system (CNS), microglia, activated monocytes, B and T lymphocytes, and dendritic cells. Calcitriol differs in structure and action from typical vitamins and is more reminiscent of a steroid hormone.
The main source of vitamin D is its formation in the skin under the influence of solar radiation (80%) and only 20% comes from food or supplements [4]. It is believed that short-term (for 10-30 minutes) solar irradiation of the face and hands is equivalent to taking 200 IU of vitamin D. The level of vitamin D synthesis in the skin is affected by latitude, time of year and day, air pollution, cloudiness, melanin content in the skin, use sunscreens, age, degree of openness of clothing.
The formation of previtamin D3 depends on the angle of incidence of sunlight. An increase in the angle of incidence due to the low position of the sun above the horizon, cloudiness, and environmental pollution determines the predominance of radiation with a longer wavelength. As a consequence, fewer ultraviolet photons reach the skin surface and stimulate vitamin D synthesis. Vitamin D production is virtually absent early in the morning and late afternoon and throughout the day during several winter months at latitudes above 35°. Therefore, living in northern latitudes is a risk factor for vitamin D deficiency. Those individuals who avoid sunlight, regardless of latitude, are also at risk for vitamin D deficiency at any time of the year. An unexpectedly high incidence of vitamin D deficiency was found in Miami [5]. It turned out that dark-skinned people need longer sun exposure to synthesize the required amount of vitamin D, since the epidermis of their skin contains large amounts of melanin, which competes with 7-dehydrocholesterol for the absorption of ultraviolet photons. In aging skin, the content of 7-dehydrocholesterol decreases, which reduces the synthesis of vitamin D in older people [6]. At age 70, vitamin D synthesis decreases by 75%. In addition, limited mobility, decreased renal production of 1,25-dihydroxyvitamin D, and reduced intake of vitamin D-fortified foods make it difficult to adequately provide vitamin D to older adults.
The level of 7-dehydrocholesterol in the skin of obese individuals is no different from that of non-obese individuals. However, subcutaneous fat creates reserves of vitamin D, due to which it is insufficient in the circulation in obese people [7].
Vitamin D levels are also affected by decreased bioavailability as a result of malabsorption, increased catabolism (when using anticonvulsants, glucocorticosteroids), decreased synthesis of 25-hydroxyvitamin D as a result of liver failure, loss of 25-hydroxyvitamin D in nephrotic syndrome, decreased synthesis of 1,25-dihydroxyvitamin D for chronic renal failure. A decrease in estrogen levels affects the activity of the enzyme α-hydroxylase, which leads to a decrease in the level of 1,25(OH)2D3 in postmenopause. Finally, women have generally lower vitamin D levels than men [8, 9]. During pregnancy and lactation, their need for vitamin D increases and is rarely compensated.
To date, debate continues regarding the optimal level of vitamin D in blood serum. Most experts believe that levels above 30 ng/mL (75 nmol/L) are adequate [10–12]. Existing recommendations declare that a serum vitamin D level of at least 75-90 nmol/L is necessary not only to optimize the metabolism of calcium and phosphorus, but also to prevent certain autoimmune diseases [13, 14]. Hypovitaminosis D is defined when the level of 25(OH)D3 in the blood serum is below 75 nmol/l (30 ng/ml), D-vitamin deficiency - at 50 nmol/l (20 ng/ml), and D-deficiency - at a level below 25 nmol/l (10 ng/ml). It is less clear what the upper level of vitamin D should be. According to the literature, a level of 150 to 200 ng/ml (375-500 nmol/l) can be considered safe [10].
In the absence of metabolic bone diseases such as rickets, osteomalacia, or osteoporosis, vitamin D deficiency is asymptomatic. Numerous studies show that the average vitamin D level in adult populations in all temperate countries where these studies were conducted is below the optimal value. For example, in the USA - 60 nmol/l (cohort of 18,558 people from 2000 to 2004) [15], 61 nmol/l - in France (cohort of 1,579 people) and in Canada [16, 17], 51 nmol/l l - in Great Britain [18]. In northern countries, vitamin D levels are even lower - for example, in Finland it was 44 nmol/l in summer and 24 nmol/l in winter (220 young men) [19]. In Russia, the first studies confirmed the presence of vitamin D deficiency both in children and adolescents (Yakutsk, Moscow, Vladivostok) and in the elderly (Moscow, Yekaterinburg) [20].
Vitamin D is the most important regulator of phosphorus-calcium metabolism, providing the necessary level of these elements for adequate osteogenesis. Calcitriol deficiency causes calcium malabsorption. A decrease in the level of calcium in the blood plasma and a decrease in the level of the active metabolite of vitamin D causes proliferation of parathyroid cells and an increase in the secretion of parathyroid hormone. Secondary hyperparathyroidism entails osteoclastic bone resorption, disruption of the processes of remodeling and mineralization of bone tissue, a decrease in its density and changes in bone architecture, which in turn leads to osteoporosis and an increased risk of fractures.
In addition to its well-known effects on calcium and phosphorus metabolism, vitamin D has other important effects, especially its anti-inflammatory, antiproliferative, and immunomodulatory effects. Considering the pathogenesis of MS, the following effects of vitamin D on the immune system may be of particular interest: the ability to modulate the differentiation and functional activity of antigen-presenting cells, thereby reducing the activity of potentially autoaggressive T lymphocytes [21-23], the ability to inhibit B-cell proliferation and T cell differentiation [24, 25], the ability to switch cytokine responses from pro-inflammatory Th1/Th17 to anti-inflammatory Th2 [26, 27], and finally, a mechanism to promote regulatory T cell differentiation and natural killer cell function [28, 29] . There is also evidence of the neuroprotective effect of vitamin D [30].
Other studies raise the question of genetic regulation of vitamin D metabolism in patients with MS. Of course, genetic factors influence vitamin D metabolism, which is regulated by more than 200 genes. The effects of calcitriol on the immune system may also be due to specific genes. Related to this is the suggestion that genes related to vitamin D synthesis may influence the risk of MS. Recently, it was shown that gene expression of the HLA-DRB1*1501 allele is modulated by vitamin D [31]. It has also been shown that the absence of functional variants in the CYP27B1
, which encodes an enzyme that converts 25(OH)D3 to its active form, is associated with an increased risk of MS [32].
The first hypotheses about the possible connection of MS with vitamin D deficiency arose from observations that confirmed that the risk of developing MS is associated with the latitude of residence [33, 34]. The prevalence of MS increases with distance from the equator. There is a hypothesis that latitude influences the risk of MS through the level of sun exposure and serum levels of vitamin D. However, recent studies show that the “latitude gradient” is being erased, which can be explained by a number of reasons, including better diagnosis of MS, changes in lifestyle, improved sanitary living conditions [35]. Most of the findings about the beneficial role of vitamin D in MS come from the fact that the risk of MS and its activity are associated with the time of year. As shown in a number of studies, people born in the spring have a significantly higher risk of developing MS than those born in the fall [36-39]. This can be partly explained by the fact that in the first case, most of the pregnancy took place during the period of low insolation (October - March), and in the second case, during the period of high insolation (April - September). Based on a study of 136 cases and 272 controls in Tasmania, the risk of MS was found to be lower in those who spent more time in the sun during holidays and weekends during childhood than in those who were protected from sun exposure ( p
<0.01) [40]. An inverse correlation between MS risk and past exposure to insolation has also been found in other studies [41, 42]. Finally, 79 pairs of monozygotic twins, one of whom had MS, were examined in the United States. Both twins differed only in the degree of sun exposure in childhood. Twins without MS had significantly more exposure to sun in the past [43]. However, all of these studies regarding the relationship between the level of insolation received in childhood and adolescence and the risk of MS in the future have a number of limitations. First, retrospective assessment of the duration of insolation in the past is difficult [36]. Also, when studying the level of insolation, it is necessary to remember that the relationship between the amount of vitamin D formed and the level of insolation is not direct. In addition, there is evidence that insolation has an immunomodulatory effect independent of vitamin D [44, 45].
Only one study has directly analyzed MS risk based on serum vitamin D3 levels in healthy individuals several years before their MS onset [46]. They studied 257 cases of MS among young American soldiers who had at least one blood serum sample taken before the onset of neurological symptoms. The control group consisted of 514 people. Whites were divided into 5 subgroups based on serum vitamin D levels: the subgroup with the highest vitamin D levels (99–152 nmol/L) had a significantly lower risk of MS than the subgroup with the lowest levels (15–63 nmol/L). , p
<0.01). For whites, the risk of MS decreased by 41% for every 50 nmol/L increase in vitamin D levels (OR=0.59; 95% CI 0.36-0.97). No statistically significant differences were obtained for the black group.
The study concluded that high vitamin D levels are associated with a lower risk of MS in white people. Based on this study, A. Ascherio and K. Munger [3] believe that almost ⅓ of MS cases can be avoided if serum vitamin D levels are maintained at around 100 nmol/L during childhood and adolescence. One of the latest studies suggests that vitamin D levels in utero
, which is entirely dependent on maternal vitamin D levels, influences the risk of subsequently developing MS [47]. A Canadian study of children experiencing a first demyelinating episode found that the risk of developing definite MS over the next 3 years was inversely and independently correlated with 25(OH)D3 levels [48].
Other studies have looked at vitamin D levels directly in MS patients. According to various researchers, the prevalence of vitamin D hypovitaminosis in patients with MS ranges from 17.0 to 86.7% [49, 50].
Thus, a relatively old American study showed that 80 hospitalized women with MS (average EDSS score 7.2) had a very low average level of 25-hydroxyvitamin D, but the low physical activity of these patients could to some extent explain the results [ 49]. At the same time, a number of studies have not found a statistically significant difference in the serum level of 25(OH)D3 in MS patients and controls. Thus, in a Finnish study of a group of 40 patients at the onset of MS, there was no difference in 25(OH)D3 levels between them and 40 control subjects if the samples were studied in winter. However, patients with MS had significantly lower levels of 25(OH)D3 in the period from June to September [51]. In another study, also conducted in Finland, vitamin D deficiency was detected with the same frequency (50%) in the patient group and in the control group. At the same time, seasonal variations in 25(OH)D3 were similar in patients and controls, but vitamin D levels were lower in patients during MS exacerbations [52].
In 2008, 267 MS patients were examined in the Netherlands. 25(OH)D3 levels were significantly lower in progressive forms of MS compared to relapsing-remitting MS. The same study showed that low 25(OH)D3 levels are associated with high EDSS scores [53]. However, it should be added that although a number of researchers have found an inverse correlation between vitamin D levels and disability scores on the EDSS scale, nevertheless, a significant proportion of patients with vitamin D deficiency have slight disability [54]. Although disability, reducing the ability to go outside, along with the systematic avoidance of heat and sun by these patients, can increase hypovitaminosis D.
J. Corrale et al. [55] conducted a study of vitamin D levels in 132 patients with different types of MS and stage of the disease and 60 controls. All subjects lived in Buenos Aires (Argentina) at latitude 34 in the southern hemisphere. The level of 25(OH)D3 was significantly lower in patients with relapsing-remitting MS (47.3±9.0 ng/ml in remission and 38.5±8.7 ng/ml in exacerbation) than in healthy controls (61.2±5.6 ng/ml). It was also demonstrated that during the period of exacerbation the level of vitamin D was significantly lower than in the remission stage. In patients with primary progressive MS, vitamin D levels did not differ from those in the control group and amounted to 52.2±6.5 ng/ml. The authors explain this difference in vitamin D levels in relapsing-remitting and primary progressive MS by the features of the pathogenesis and morphology of these two fundamentally different forms of MS.
A study of vitamin D levels in MS patients in Russia using the example of the Sverdlovsk region also established a high incidence of vitamin D hypovitaminosis both among MS patients and healthy volunteers. However, in patients with MS, vitamin D deficiency was more common and more pronounced. The magnitude of the decrease in 25(OH)D3 levels did not correlate with any of the MS characteristics studied, but was more pronounced in younger patients. There was a tendency for vitamin D3 concentrations to decrease during MS exacerbations [56].
As is known, depression and fatigue are common symptoms in MS; they are also associated with hypovitaminosis D. A study of depression and fatigue in 59 MS patients was conducted in the Netherlands in 2010 [57]. Vitamin D levels were negatively correlated with depression levels ( r
=–0.326,
p
=0.006). No such correlation was found for fatigue. The authors conclude that vitamin D deficiency may contribute to the presence of depressive symptoms in MS, although this requires confirmation.
Recent studies demonstrate a clear connection between MS activity and vitamin D concentrations. Thus, 2 studies showed that each increase in 25(OH)D3 levels by 10 nmol/l led to a decrease in the frequency of exacerbations by 11.0 and 13.7%, respectively [58, 59]. Another study [60] found that for every doubling of serum vitamin D levels, the incidence of exacerbations decreased by 27.0%.
When studying the level of vitamin D in the cerebrospinal fluid in patients and controls, no significant differences were obtained, and no differences were found in studies in the stages of remission and exacerbation [61].
Experimental studies show that vitamin D prevents the development of experimental autoimmune encephalomyelitis and markedly improves clinical symptoms in affected mice [62–65]. However, this effect is more pronounced in females, possibly due to the enhanced effect of vitamin D by estrogens [66].
To date, several clinical studies have been conducted on the use of vitamin D in the treatment of MS, although the quality of some of them is questionable.
In one of the early studies, P. Goldberg et al. [67], using vitamin D (5000 IU per day in the form of cod liver oil) in 16 patients for 2 years, noted a 60% reduction in the frequency of MS exacerbations. A Canadian study demonstrated the use of high doses of vitamin D3 (cholecalciferol, 14,000 units per day) over a long period (6 months - 1 year) without the development of hypercalcemia or other significant side effects, despite achieving vitamin D3 levels of about 400 nmol/L. After 1 year of such treatment, there was a 41% reduction in the number of exacerbations and a significant improvement in the EDSS scale in treated patients [68].
Two recently published randomized, double-blind, placebo-controlled studies conducted in Finland [69] and Norway [70] using 20,000 IU vitamin D per week gave conflicting results. In a Finnish study, the mean 25(OH)D3 value in patients receiving vitamin D for 1 year in addition to interferon β increased to 110 nmol/L, and patients in the drug group had significantly fewer Gd-accumulating lesions. At the same time, there was a tendency to reduce the accumulation of disability and improve walking parameters. The annual rate of exacerbations was the same in both the drug and placebo groups.
In a 96-week Norwegian study, there was no difference between the groups in the annual frequency of exacerbations, EDSS, and MSFC scales, although the median D3 concentration in the group receiving vitamin therapy reached 121 nmol/l.
Due to the rather uncertain results of clinical studies published to date, it is impossible to answer the question of whether vitamin D can be used to treat MS. Well-designed clinical studies are currently underway that may help clarify this issue. Regarding the safety of high doses of vitamin D, this data already exists. Treatment of MS patients with high doses of vitamin D is safe [71–73].
Thus, numerous studies show that the majority of patients with MS have vitamin D deficiency. An association has been identified between vitamin D deficiency and disease process activity. Further research is needed to definitively prove the link between vitamin D levels in the body and the risk of developing MS. Although the effect of vitamin D in the treatment of MS is currently unproven, from a purely medical point of view it cannot be ignored that vitamin D deficiency is generally detrimental to the health of these patients. Although there are no practical recommendations, it is nevertheless advisable to screen individuals at risk for MS for vitamin D levels in their blood serum. In case of vitamin D deficiency, its level should be increased to 30-40 ng/ml (75-100 nmol/l).
Drugs
Glucocorticoids
These are synthetic analogues of adrenal hormones - Prednisolone, Methylprednisolone, Dexamethasone. These drugs for multiple sclerosis block certain biologically active substances of the cytokine class and reduce the production of T-lymphocytes. Thus, glucocorticoids inhibit inflammatory and autoimmune processes. In addition, these drugs reduce the permeability of vascular walls and the BBB. Glucocorticoids for multiple sclerosis are used in pulse therapy mode - in large doses over short periods of time.
ACTH
Adrenocorticotropic hormone is secreted by the anterior pituitary gland and stimulates the production of its own glucocorticoids by the adrenal cortex. Thus, the effect of ACTH and its synthetic analogues (Synacten, Tetracosactide) is similar to the drug of the previous group, but has less risk of side effects (ulceration, hypercortisolism). Although in this case, disturbances in water-salt metabolism and increased blood pressure are possible.
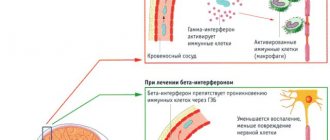
Synthetic beta interferons
(“Avonex”, “Rebif”, “Betaferon”) have an immunomodulatory effect, inhibit autoimmune processes, reduce the activity of T-lymphocytes, and prevent the penetration of cytokines through the BBB. In addition, beta interferons are effective in the initial stages of viral infections, one of the causes of multiple sclerosis.
Copaxone
This drug has immunomodulatory properties and is specially designed for the treatment of multiple sclerosis. It is a sequence of amino acids glutamine, lysine, alanine, tyrosine. Its chemical structure is similar to myelin.
Angioprotectors and antiplatelet agents
These drugs (“Curantil”, “Pentoxifylline”) prevent red blood cells from sticking together, improve blood flow and strengthen the vascular wall.
Proteolytic enzyme inhibitors
When administered intravenously, Contrikal and Gordox inhibit enzymes of the protease class, which destroy protein structures and participate in inflammatory processes. Plasmapheresis, a method of hardware extracorporeal (outside the body) blood purification, gives good results. During plasmapheresis, antigenic complexes, biologically active substances that trigger autoimmune processes, are removed along with plasma. Not so long ago, the drug Anti-LINGO-1 was developed in the USA, which accelerates remyelination. Now this drug is in the clinical trial phase, but perhaps it will still say a new word in the treatment of multiple sclerosis.
Common symptoms and signs of multiple sclerosis
It is immediately worth noting that the course of the disease for each patient is purely individual. In one person, multiple sclerosis can manifest itself very violently, while in another it can be completely unnoticeable. But general symptoms of sclerosis include: