Meningoencephalitis is a disease of a neuroinfectious nature that occurs with combined damage to the brain matter and membranes.
The term “meningoencephalitis” means inflammation of the cerebral membranes (“meningea”) and the substance (“encephalon”) of the brain, which occurs simultaneously. Such a process can occur independently or be a consequence of the spread of a pathological process in the body. With secondary damage to the brain matter, the disease acts as a complication of meningitis, and when inflammation spreads to the meninges, it becomes a complication of encephalitis.
The main causes of meningoencephalitis in adults are:
- Entry of pathogens (viruses, bacteria, protozoa, pathogenic fungi) into the nasopharynx. Infection occurs by airborne droplets and alimentary methods (through contaminated food, water). Penetration into the brain occurs through the hematogenous route, resulting in inflammatory changes in tissues, leading to the development of meningoencephalitis;
- Insect bites. Transmissible transmission is the main route for a number of viral meningoencephalitis and encephalitis, such as Japanese mosquito encephalitis, tick-borne encephalitis, St. Louis encephalitis). When bitten, the pathogen enters the bloodstream and enters the cerebral tissue, thus provoking the disease;
- The presence of various infections in the body. Hematogenous spread of bacterial infections occurs in the presence of tuberculosis (tuberculous meningoencephalitis), syphilitic foci, purulent processes in the paranasal sinuses, maxillofacial area, and chronic purulent otitis media. Viral meningoencephalitis can occur as a result of a complication of acute respiratory viral infection;
- Traumatic brain injuries. Infection by contact occurs as a result of an open injury with a violation of the integrity of the cranial bones. According to statistics, post-traumatic meningoencephalitis is observed in 1.5-4% of patients with traumatic brain injury;
- Vaccination. The introduction of a live vaccine in the presence of a weakened immune system is dangerous for the development of an infectious process in the body. Penetration of pathogens occurs through the blood-brain barrier, which leads to the appearance of meningoencephalitis;
- The presence of immunodeficiency;
- Immaturity of the immune system.
At the Yusupov Hospital, patients with meningoencephalitis are provided with effective, qualified medical care. Doctors use modern equipment to diagnose and treat the most dangerous diseases. At the Yusupov Hospital, specialists are interested in the speedy recovery of patients, and therefore will do everything to speed up the recovery process. Thanks to cooperation with European doctors, it is possible to achieve significant positive results.
Features of the pathology
Features of the disease lie in the nature of its development. This form of meningitis develops rapidly, but without pronounced symptoms. Symptoms of this disease:
- nausea;
- vomit;
- headaches without precise localization;
- general malaise;
- increase in body temperature.
Meningeal complications are not observed in the serous form of the disease. The pathology does not provoke impaired thinking, confusion and other symptoms characteristic of meningitis.
Diagnosis of meningoencephalitis
Diagnosis of patients begins with collecting an anamnesis of life and illness, the doctor learns about a current or recently suffered infectious disease, a history of traumatic brain injuries, the fact of vaccination, insect bites, etc. Further diagnostic manipulations are as follows:
- Neurological examination. Meningeal symptoms are identified, the patient’s state of consciousness and focal neurological status are assessed. The data obtained will indicate whether the brain membranes and substance are involved in the pathological process;
- Laboratory research. Bacterial meningoencephalitis will be characterized by leukocytosis and accelerated ESR. Also, to determine the pathogen, blood cultures are performed for sterility and PCR analysis;
- Computed and magnetic resonance imaging. They allow you to assess the condition of the brain, identify thickening and compaction of the cerebral membranes, and changes in brain tissue. In the presence of parasites, heterogeneous round lesions with ring-shaped enhancement along the periphery will be visualized on the images;
- Lumbar puncture. With its help, the cerebrospinal fluid is examined, which, with purulent inflammation, will be cloudy and have a sediment in the form of flakes, with a serous lesion - transparent, hemorrhagic - with an admixture of blood;
- Stereotactic brain biopsy. Allows you to diagnose parasitic meningoencephalitis and exclude a malignant process.
At the Yusupov Hospital, specialists carry out differential diagnosis of meningoencephalitis with brain tumors, strokes, toxic damage to the central nervous system, and a progressive degenerative process. We use innovative equipment that will allow you to perform all diagnostic measures quickly and efficiently.
ICD-10 code
Serous meningitis is most often caused by viruses. However, inflammation can begin due to bacterial or fungal infection of the meninges. Due to the fact that serous meningitis can be caused by various pathogenic factors, it does not have a precise classification according to ICD-10 and is classified as “other viral meningitis”.
The disease is listed under code A87.8, where A87 is a classification of viral brain lesions, and the number 8 means viral inflammation of the brain, provoked by the action of other viruses not included in the classifier.
If the inflammation is caused by a bacterial lesion, it is classified as G00.8. This labeling describes purulent meningitis (class G00), provoked by other bacteria (this is indicated by the number 8 in the code).
Treatment of pathology
Treatment of the disease begins after determining the cause of the inflammatory process. If meningitis is caused by a virus, antiviral therapy is prescribed. For bacterial diseases, antibiotics are used, and for fungal infections, special antimycotics are used to combat a specific type of fungus.
In addition to treatment aimed at eliminating the cause of the disease, symptomatic therapy is used to improve the patient’s well-being as soon as possible. Viral and bacterial damage to the brain can be accompanied by an increase in temperature, so antipyretic medications are additionally prescribed. Nootropic medications are often used to improve cerebral circulation. Therapy must be supplemented by taking vitamin complexes containing B vitamins.
With timely treatment, the pathology resolves successfully without causing complications.
Cryptococcosis of the central nervous system
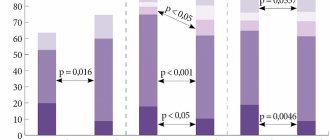
Over the past decades, fungal diseases have not lost their relevance. Among all invasive mycoses, cryptococcal infection occupies one of the most significant places, as it is a life-threatening disease. Even with timely treatment, mortality ranges from 10% to 25%, and in developing countries reaches 90%. Every year, about 1 million cases of cryptococcosis are recorded worldwide. In Russia, at this stage, there is no mandatory registration of deep mycoses. The true incidence is not known, but according to the North-Western State Medical University named after. I.I. Mechnikov in St. Petersburg, there was a significant increase in cryptococcosis with a fatal outcome in the period from 2002 to 2010 [1–5].
The genus Cryptococcus includes more than 70 species of basidiomycete encapsulated yeasts, of which Cryptococcus neoformans and Cryptococcus Gatti are of medical importance. Cryptococcus Gatti is most common in countries with tropical climates; Cryptococcus neoformans dominates in Europe and Russia. The pathogenicity factors of cryptococcus include its ability to grow at a temperature of 37 °C (i.e., at the body temperature of warm-blooded animals), as well as the ability to form a polysaccharide capsule, which is a protection against phagocytosis and humoral immunity factors. Cryptococci synthesize a number of enzymes, including urease and phospholipase. Urease promotes yeast adhesion to endothelial cells and forms mucous “cysts” without initiating an inflammatory response. Phospholipase destroys human cell membranes and facilitates attachment to the pulmonary epithelium. The ability of fungi to form melanin serves as protection against antibody-mediated phagocytosis [6–10].
People with disorders of the immune system are susceptible to clinically expressed forms of cryptococcosis. Most often these are patients with acquired immunodeficiency syndrome (AIDS), leukemia, sarcoidosis, lymphoma, as well as patients after organ transplantation and patients receiving large doses of cytostatics or corticosteroids. Infection occurs by inhalation; the lungs are the organ of primary localization of the pathogen, where it can remain latent for a long time. When the functioning of the immune system is disrupted, infection is activated [11, 12].
The clinical picture of cryptococcosis depends on the localization and extent of the process. According to ICD-10, pulmonary cryptococcosis, cerebral cryptococcosis, skin cryptococcosis, and bone cryptococcosis are distinguished. Pulmonary cryptococcosis may be asymptomatic or accompanied by a cough with mucous sputum, mild fever, malaise, and rarely shortness of breath. X-ray visualizes nodular infiltrates and confluent foci located subpleurally. Cryptococcal pneumonia is detected in 1/3 of AIDS patients. The disease is characterized by rapid progression with the development of respiratory distress syndrome and acute respiratory failure. Cryptococcosis of the skin occurs in 10–15% of cases. It is characterized by the appearance of papules, which transform into a plaque with compaction and subsequent ulceration in the center of the element. They are most often localized on the scalp and face. Cryptococcosis of bones is characterized by osteolysis, the incidence is 5%. The bones of the pelvis, spine, skull, and ribs are affected [13].
The most common clinical form is cerebral cryptococcosis, which in 80–90% occurs in the form of meningoencephalitis. In the central nervous system (CNS), the pathogen disseminates hematogenously. The target for the pathogenic effects of cryptococci is the endothelium of the microvasculature. The main way the pathogen penetrates into the brain is by destroying the vessel wall. As a result, a violation of microcirculation occurs, which entails neuronal degeneration and the development of foci of necrosis. Due to sharply increased vascular permeability, fluid accumulates in the brain matter. Histologically, proliferation of yeast-like fungi with infiltration represented predominantly by lymphocytes, histiocytes, a small number of plasma cells, and single neutrophils is noted. At autopsy, damage to the meninges is characterized as serous-productive meningitis with pinpoint hemorrhages in the soft and dura mater. The shells are thickened, cloudy, with multiple small tubercles on the surface (clusters of fungi). The pathological process can also involve the basal surface of the brain. The cause of death is cerebral edema with dislocation of stem structures [13, 14].
The dominant complaint is a constant headache of a diffuse nature, the intensity of which gradually increases. Nausea and vomiting are observed in 40% of patients. The febrile reaction is not constant, body temperature fluctuates widely from 37.2 to 39.5 ° C. Meningeal symptoms (stiff neck muscles, Kernig's sign, Brudzinski's sign) are often absent or questionable. Convulsions and impaired consciousness occur in isolated cases and, as a rule, in the late stages of the disease. Some patients may exhibit congestive optic discs, blurred vision, and focal neurological symptoms. When examining the cerebrospinal fluid (CSF), inflammatory changes are mild. Usually there is an increased protein content and lymphocytic two-three-digit pleocytosis. A progressive decrease in glucose levels is characteristic [7, 12, 13, 15, 16].
To diagnose cryptococcal lesions of the central nervous system, the main method is microbiological examination of the CSF. For microscopy, smears are filled with 1–2 drops of ink. The capsule of Cryptococcus neoformans is not completely stained and forms specific rims. Cryptococcus cells have a spherical or ellipsoid shape. The diameter varies from 5 to 7 microns, rarely from 2 to 15 microns. The capsule is well stained using the Mowry method with alcian blue to a blue-green color. This staining method allows visualization of phagocytosed cryptococci. The effectiveness of this method reaches 97% [5, 17].
To confirm the diagnosis, the culture method is crucial. Cryptococcus neoformans grows from 48 to 72 hours, on wort agar or Sabouraud medium, at a temperature of 37 °C. Externally, these are white, smooth, shiny, mucous-stringy colonies. Obtaining a culture of cryptococcus allows one to determine the sensitivity of the isolated strain to antifungal drugs. Microbiological diagnostics are also used for other forms of cryptococcal infection. Substrates for research are blood, sputum, and skin secretions. An additional method is latex agglutination. The specificity and sensitivity of standard tests exceed 90%. In recent years, polymerase chain reaction (PCR) has been increasingly used for diagnostic purposes; the value of this method lies in the fact that it allows a diagnosis to be made in the shortest possible time [14, 16, 17].
Neuroimaging methods (computed tomography and magnetic resonance imaging) in some cases allow us to obtain additional data on the nature of the process. 34% have atrophy of the cerebral cortex, 11% have damage to the brain substance of both diffuse and focal nature (cryptococcoma). Hydrocephalus is detected in 9%; in 50% no pathological changes are detected [13, 17].
To treat meningoencephalitis of cryptococcal etiology, it is necessary to use antimycotic drugs that penetrate the blood-brain barrier (BBB) in therapeutic concentrations. These include flucytosine, amphotericin B, and fluconazole. Flucytosine has both fungistatic and fungicidal effects. By integrating into the ribonucleic acid (RNA) of the pathogen, it disrupts the formation of proteins, and also by suppressing the activity of thymidylate synthetase, it prevents the synthesis of fungal deoxyribonucleic acid (DNA). Its concentration in the CSF is 75% of the concentration in plasma. Amphotericin B has a largely fungistatic effect. The mechanism of action is the ability to bind to ergosterol in the cell membrane of the pathogen. Pores form in the membrane, the barrier function is disrupted, which entails the loss of cellular structures and the death of the fungus. Its concentration in the CSF when administered intravenously is 5%, so endolumbar administration is preferable. The combination of these two drugs gives a better therapeutic effect, in contrast to monotherapy. In addition, this makes it possible to reduce the dose of amphotericin B and, thereby, reduce its toxic effect and shorten the duration of treatment. The use of combination therapy can prevent or delay the development of pathogen resistance. The sensitivity of Cryptococcus neoformas to amphotericin B is 68%, flucytosine 54%. Fluconazole penetrates equally well into the biological environment of the body, its concentration in the CSF is about 85%. The fungistatic effect of this drug is the ability to inhibit the synthesis of ergosterol in the fungal membrane. Sensitivity to it is 74% [16, 22, 23].
Currently, the following treatment regimen is used: amphotericin B 0.7–1.0 mg/kg/day in combination with flucytosine 100 mg/kg/day for two weeks, followed by fluconazole 800–400 mg/day for at least 10 weeks . If the patient's condition does not improve in the first two weeks of treatment, then treatment with amphotericin B is extended. For persons with a persistent risk factor for recurrent infection (patients with AIDS), maintenance therapy with fluconazole 200–400 mg/day is recommended for 6 months to a year. The main side effect of this treatment is kidney toxicity, which can occur in up to 80% of patients. Therefore, it is advisable to use the liposomal drug amphotericin B (Ambisome), which has less toxicity. Endolumbar administration of amphotericin B at a dose of 0.25–1.0 mg 2–4 times a day is also possible. If disease relapse occurs, it is recommended to use amphotericin B or liposomal amphotericin B at a dose of 1 mg/kg/day for 4–10 weeks. As maintenance therapy, fluconazole 800–1200 mg/day for at least 10–12 weeks. During treatment, regular monitoring of the sensitivity of cryptococcal strains to antimycotic drugs is necessary to adjust therapy in case of pathogen resistance [5, 16, 24].
In addition to etiotropic therapy, correction of intracranial pressure is necessary, since its persistent increase indicates the possibility of developing cerebral edema and swelling with dislocation, which is the main cause of mortality. This complication is also possible against the background of specific therapy, since lysis of cryptococcus leads to the release of toxic cell components that contribute to increased vascular permeability. Therefore, systematic dehydration therapy using loop and osmotic diuretics is necessary. When CSF pressure is above 250 mm. Art. Daily punctures are recommended until the indicators decrease. A persistent increase in CSF pressure requires permanent drainage (ventriculoperitoneal shunt) [5].
The effectiveness of treatment is judged by the clinical condition of the patient and the improvement in the composition of the CSF. First of all, normalization of glucose levels, negative results from microscopic and bacteriological examination of the cerebrospinal fluid, as well as from PCR results. A decrease in microbial load by 2–3 orders of magnitude within two weeks is a good prognostic sign and criterion for the adequacy of therapy [2, 16].
Mortality in cryptococcosis without the use of antimycotic therapy reaches 100%. It is due to: late diagnosis, resistance of the pathogen, the possibility of developing ONGM against the background of antimycotic therapy, low adherence of patients to treatment or refusal of it (primarily HIV-infected) [16, 25].
Thus, the problem of cryptococcal damage to the central nervous system is becoming increasingly relevant. The low information content of the clinical picture of the disease requires doctors to carry out early examination of the CSF when neurological symptoms appear, especially prolonged headache in patients at risk, even in the absence of meningeal symptoms. During the treatment process, increased attention to the patient’s condition (the possibility of sudden development of ONGM), microbiological control of the CSF with determination of the sensitivity of the pathogen (the possibility of developing resistance) and determination of the microbial load by PCR as the most reliable method of assessing the effectiveness of treatment is necessary.
Literature
- Elinov N.P., Bosak I.A. Past and present of Cryptococcus neoformans (Sanfelice) Vuillemin (1901) as an object of study of a potentially dangerous pathogen for humans // Problems of Med. mycology. 2006. T. 8, No. 2. P. 47–51.
- Klimko N. N. Mycoses: diagnosis and treatment. A guide for doctors. M., 2007. 336 p.
- Vasilyeva N.V. Cryptococci and cryptococcosis at the present stage // Problems. honey. mycology. 2002. T. 4, No. 2. P. 45–46.
- Lesovoy V. S., Lipnitsky A. V. Mycoses of the central nervous system (review) // Problems of medicine. mycology. 2008. T. 10, No. 1. P. 3–6.
- Klimko N. N. Mycoses of the central nervous system. St. Petersburg: Infectious diseases: problems, achievements and prospects, 2011.
- Voelz K. Macrophage-Cryptococcus interactions during cryptococcosis // PhD Thesis. 2010.
- Vasilyeva N.V. Pathogenicity factors of Cryptococcus neoformans and their role in the pathogenesis of cryptococcosis. Diss. ... doc. biol. Sci. St. Petersburg, 2005.
- Vasilyeva N.V., A.A. Stepanova, I.A. Sinitskaya. Ultrastructure of capsules of mature cells of Cryptococcus neoformans strains in vitro and in vivo // Problems of medical mycology. 2006. T. 8, No. 2. P. 25.
- Charlicr C. Capsule structure changes associated with Cryptococcus neoformans crossing of the blood-brain barrier/C. Charlier, F. Chretien, M. Baudrimont, E. Mordelet, O. Lortholary, F. Dromer // Am. J. Pathol. 2005. V. 166, No. 2. P. 421–432.
- Romani L. Immunity to fungal infection // Nat. Rev. Immunol. 2004. Vol. 4. P. 1–23.
- Del Poeta M. Role of phagocytosis in the virulence of Cryptococcus neoformans // Eukaryotic cell. 2004. Vol. 3. P. 1067–1075.
- Badley JW, Perfect JR, Oster RA Pulmonary cryptococcosis in patients without HIV infection: factors associated with disseminated disease // Eur. J. Clin. Microbiol. Infect. Dis. 2008. Vol. 27, No. 10. P. 937–943.
- Sergeev A. Yu., Sergeev Yu. V Fungal infections. A guide for doctors. M.: Binom-press LLC, 2003. 440 p.: ill.
- Khmelnitsky OK, Khmelnitskaya N. M. Pathomorphology of human mycoses St. Petersburg: Publishing house SPbMAPO, 2005. 432 p.
- Bartlett J., Gallant J., Pham P. Clinical aspects of HIV infection. 2009–2010. M., 2010. 459 p.
- Vengerov Yu. Ya., Volkova O. E., Safonova A. P., Svistunova T. S., Vorobyov A. S., Marinchenko M. N., Martynova N. N. Clinic and diagnosis of cryptococcal meningoencephalitis in HIV-infected patients infection. Materials of the V Annual All-Russian Congress on Infectious Diseases. 2013. P. 85.
- Arabian R. A., Klimko N. N., Vasilyeva N. V. Diagnosis of mycoses. St. Petersburg: Publishing house SPbMAPO, 2004. 186 p.
- Larsen RA, Bauer R, Thomas AM et al. Amphotericin B and fluconazole. A potent combination therapy for cryptococcal meningitis // Antimicrob. Agents. Chemother. 2004. Vol. 48, No. 3. P. 985–987.
- Barchiesi F., Spreghini E., Schmizzi A. Posaconazole and amphotericin B combination therapy against Cryptococcus neoformans infection // Antimicrob. Agents. Chemother. 2004. Vol. 48, No. 9. P. 3312–3316.
- Saag MS, Graybill RJ, Larsen RA et al. Practice guidelines for the management of cryptococcal disease // Clin. infection Dis. 2000. Vol. 30, No. 3. P. 710–718.
- Berard H., Astoul P., Frenay C. et al. Disseminated histplasmosis caused by Histoplasma capsulatum with cerebral involvement occurring 13 years after the primary infection // Rev. Mal. Respira. 1999. Vol. 16, No. 5. P. 829–831.
- Haynes RR, Connolly PA, Durkin MM et al. Antifungal therapy for central nervous system histoplasmosis, using a newly developed intracranial model of infection // J. Infec. Dis. 2002. Vol. 185, No. 9. P. 1830–1832.
- Saccente M, McDonnell RW, Baddour LM et al. Cerebral histoplasmosis in the azole era: report of four cases and review // South J. Med. 2003. Vol. 96, No. 4. P. 410–416.
- Sergeev A. Yu. Evolution of antimycotics and revolutions in the treatment of mycoses // Advances in medical mycology. 2002; 1: 111–112.
O. E. Volkova1 Yu. Ya. Vengerov, Doctor of Medical Sciences, Professor
GBOU VPO MGMSU im. A. I. Evdokimova Ministry of Health of the Russian Federation, Moscow
1 Contact information