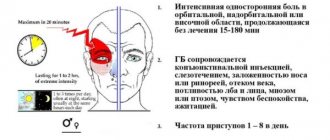
Turn back the clock: new frontiers in the treatment of multiple sclerosis
– Multiple sclerosis is a fairly common neurological disease throughout the world. What is the situation in Russia?
– I wouldn’t say that we are somehow different from the rest of the world; we have approximately the same age range of patients. In terms of incidence, we belong to a medium-risk zone, we have from 10 to 50 cases per 100 thousand population, but in different regions the situation is slightly different. Official current statistics, unfortunately, are known only to the Ministry of Health of the Russian Federation, but at a recent meeting of the Federation Council, representatives of the department reported that we already have more than 85 thousand patients in the federal register. Although the medical community assumes that there should be more than 100 thousand patients.
– How is the profile patient audience classified?
– It is customary to distinguish three types of multiple sclerosis: relapsing-remitting, secondary progressive and primary progressive. In 70–85% of patients, the disease debuts with a relapsing course, which subsequently becomes secondary progressive. Approximately 10–15% are patients with a primary progressive course. There are no official statistics on the primary progressive course in Russia yet, because there was no targeted therapy for these patients until 2021. Therefore, as a rule, their share turned out to be underestimated, somewhere between 2–5% of those officially observed. Now, since targeted therapy has appeared, the approach to both register maintenance and diagnosis has changed. We are striving for earlier diagnosis and greater consideration of these patients, because now there are solutions that we can offer them as therapy. Accordingly, we will gradually get closer to identifying 10% of patients with the primary progressive form and will approximately reflect this group statistically. If we take all patients divided by lobe, it is difficult to say unambiguously, but probably about 70% will be relapsing-remitting, 20% secondary-progressive and about 10% primary-progressive patients.
– What are the scenarios for the development of the disease?
– Patients with a remitting course have the most favorable scenario, because their disease progresses with exacerbations and remissions. Accordingly, during an exacerbation, some new symptoms appear. Some or all of them may regress or may leave behind neurological deficits. But during the period of remission, it is considered that the patient’s condition is stable, that is, there is no increase in symptoms or increase in disability. If the frequency of exacerbations is high or they generally persist, then, of course, this patient may at some point acquire signs of disability. In the secondary progressive type, which replaces the remitting phase of the disease, the disease does not progress from the very beginning. This is precisely the patient who initially had an undulating course of the disease, and then began to show signs of a steady increase in neurological deficit and, accordingly, worsening disability. At the same time, he may continue to have exacerbations, but progression does not depend on them. In some patients, exacerbations at this stage of the disease are no longer observed and only an increase in disability is observed. With the primary progressive type, it is believed that immediately, from the very onset, there is a steady increase in neurological deficit. That is, the symptoms do not regress, but only new manifestations of the disease appear.
– What are the current approaches to treating patients with relapsing-remitting multiple sclerosis?
– Therapeutic approaches remain unchanged. We have pathogenetic therapy, and there is symptomatic therapy. Pathogenetic includes hormonal therapy for exacerbations and immunomodulatory therapy aimed at preventing exacerbations, delaying the progression and increase of changes in brain tissue. For patients with relapsing-remitting multiple sclerosis, the range of drugs is the widest. Almost every year a new drug is released that adds treatment options. Tactically, among all patients with a relapsing course, patients with a rapidly progressing scenario and with highly active multiple sclerosis began to be distinguished. These are those patients in whom either each exacerbation leaves a neurological deficit, and thus the patient’s condition worsens with each new exacerbation, or those who, in principle, during the year, despite receiving any therapy, experience two or more exacerbations, that is It is difficult to keep them simply in a state of remission. When monitoring such patients, a more aggressive strategy is used: either early escalation of therapy - they are transferred from the first line to the second, or the doctor immediately has the right to begin treatment with second-line drugs.
– At what point are patients with multiple sclerosis prescribed drug therapy?
– In general, world practice, as well as Russian practice, is as follows: as soon as a patient is diagnosed with multiple sclerosis, doctors focus on early initiation of therapy. In our country, after a diagnosis is established, personalized information about the patient is entered into the federal register of the Ministry of Health of the Russian Federation, and the patient acquires the right to receive drug therapy free of charge. Another thing is that not all patients agree to treatment that lasts for years; some prefer to be monitored before starting to take medications. In any case, we try to select the optimal treatment option for everyone.
– How then is the effectiveness of the prescribed drug determined?
– Three criteria are used here: firstly, clinical observation – exacerbations persist or are absent; secondly, a neurological examination with assessment of the condition according to the international disability scale EDSS - whether or not there is an increase in neurological deficit; and thirdly, dynamic MRI of the brain/spinal cord allows one to assess the activity of the process in the form of the appearance of new foci or contrasting foci.
– Given the emergence of new drugs, do doctors’ expectations of therapy change?
– Of course, we want, in addition to influencing quantitative changes in brain tissue, to also have the opportunity to inhibit brain atrophy, the rate of which in our patients is higher than age-related physiological atrophy. This is one of the new requirements. And the second is the ability to influence neurodegeneration. In other words, it would be ideal if it were possible to reduce persistent neurological deficits and reverse the process. But so far there are no drugs that can meet these expectations.
– Do you work with the drug alemtuzumab (Lemtrada ® ) and how do you evaluate the practice of its use?
– I have extensive experience in using this drug, I am familiar with it from the development stage and have been observing patients on therapy since 2003 as part of clinical trials. I have quite good impressions, although I can immediately say that alemtuzumab is a complex drug, so it is not indicated for all patients in terms of safety profile.
In the most recent clinical trial of the drug, nine years of data are now available, 49% of patients in the CARE-MS II clinical trial experienced improvement and maintained it over that time. The maximum follow-up period for my patients treated with alemtuzumab was 15 years, but that study has now been completed and the drug has already been registered. Despite this, the developer continues to collect data regarding both the effectiveness and safety profile during phase III clinical trials. Alemtuzumab therapy differs from other immunomodulatory drugs in its cyclic dosing regimen. The first treatment course of therapy consists of two treatment cycles with an interval of a year. There are some patients in my cohort who, after receiving these two treatment cycles, no longer required re-introduction of the drug throughout the study follow-up period. And there are patients who, in some cases three years later, in others five or six years later, still had a “surge” in disease activity. They carried out another treatment cycle and thanks to this maintenance cycle they were able to keep the patient in a stable condition. Therefore, it cannot be said that 100% of patients were stable based on the results of the first starting cycle. But according to clinical studies conducted by the developer, approximately 55% of those observed did not really need to re-administer the drug and at the same time maintained a stable condition. That is, they received two treatment cycles and then everything was fine.
– For what group of patients is the drug recommended?
– Now the drug has updated instructions, which clearly define the patient profile. These are patients with ongoing disease activity on the background of first-line immunomodulatory therapy (highly active disease) or patients with rapidly progressive multiple sclerosis, who, as I have already said, have two or more exacerbations leading to an increase in neurological deficit. Such patients, in principle, according to world statistics, constitute 15–20% of all patients with the remitting type of the disease. This is the target patient population for this type of therapy.
– One of the new criteria for the effectiveness of the drug is confirmed reduction in disability (CDI). What current data does the medical community have on this indicator?
– Naturally, we doctors dream of reducing disability. Data from studies of alemtuzumab suggest that such a result is possible. But we must understand that this effect of reducing disability is not achievable in all patients. It is probably possible to obtain this result, and it will be lasting, if the patient’s profile is correctly determined and, most importantly, therapy is started in a timely manner. Turning back the clock is probably possible if therapy is started in the first three to five years from the onset of the disease.
– When and how is a confirmed reduction in disability assessed?
– Assessment is carried out using the Expanded Disability Scale EDSS. The level of disability corresponds to a certain score, which can increase or decrease. The higher the score, the greater the patient's level of disability. After a neurological examination, the doctor can assess the level of EDSS and note whether there is an increase in it, which tells us about the progression of the disease, or a decrease, indicating an improvement in the patient’s condition. Imagine you have some symptoms, and then their severity decreased, or they regressed, they are gone.
– The drug Lemtrada ® (INN alemtuzumab) was included in the list of 14 high-cost nosologies in 2021, how is this decision assessed by the medical community?
- Of course, it’s positive. This makes it possible to provide the drug to as many patients as this therapy is indicated for. Each subject of the Russian Federation determines the need for the drug itself. Previously, the availability of this therapy was limited by the financial capabilities of the subject; now these drugs are centrally purchased by the Ministry of Health.
The material was prepared with the support of the representative office of Sanofi-Aventis Group JSC (France)
MAT-RU-2001393
One among one's own
Methylprednisolone was one of 42 drugs, the shortage of which was announced by the All-Russian Union of Patients at the end of 2021 in a letter to Russian President Vladimir Putin, the government, the Ministry of Health and the Ministry of Industry and Trade. Izvestia wrote about this. VSP received a response from the Ministry of Health, dated January 22, only on February 18.
Drug famine: Russia announced a shortage of 42 drugs
The All-Russian Patients' Union appealed to the authorities with a list of measures to eliminate the problem.
Regarding methylprednisolone, it says that the drug is included in recommendations for the treatment of a new coronavirus infection, which is why demand for it has increased significantly. At that time, the ninth version of the COVID-19 treatment manual was in effect. Now the tenth has already been released, and the drug remains in it.
The Ministry of Health of the Russian Federation explained to Izvestia that methylprednisolone in dosage form for intravenous and intramuscular administration is produced under the trade names “Metypred Orion” - (Finland), “Ivepred” - (India), “Solu-Medrol NovaMedica” - “Pfizer MFG. Belgium N.V.” (Belgium)
In the department's response to the All-Russian Patients' Union, it is reported that in order to prevent defects in drugs used in the provision of medical care in accordance with temporary guidelines, government decree No. 3353-r dated December 15, 2020 was issued. It provides for the import of unregistered drugs into Russia. Since January 4, 1.8 million bottles have been sent to the subjects under the international nonproprietary name (INN) of methylprednisolone.
No injection
Photo: Depositphotos
Inhalation delay: cystic fibrosis patients are afraid of being left without treatment
In some regions, doctors refuse to provide intravenous therapy at home
In addition, according to government decree No. 441 dated 04/03/2020, in December 2021, permission was received for the temporary circulation of methylprednisolone (Pfizer Innovations) 500 mg bottle - 3962 packs, and methylprednisolone (Pfizer Innovations) 1000 mg bottle - 4300 packs.
“The above-mentioned drugs were supplied to Russia by Pfizer Innovations LLC as part of a charitable supply to the First Moscow State Medical University named after I.M. Sechenov Ministry of Health of the Russian Federation for the treatment of patients with new coronavirus infection (COVID-19),” the document says.
The Ministry of Health additionally informed Izvestia that Orion Corporation is considering the issue of adding an additional production site, which will increase the volume of supplies of the drug in injectable form. The company informed the Russian Ministry of Health about the planned import of about 80 thousand packages in April 2021.
In addition, it notified the Russian Ministry of Health about the launch of 28 thousand packages on the country’s pharmaceutical market in February 2021. Of these, 18.5 thousand packages have already been put into civilian circulation. The company also announced possible additional deliveries of more than 150 thousand packages by the end of April 2021.
More expensive than life: 69% of orphan patients complain of problems with medications
Families of patients are forced to go to court to obtain medications