Mirtazapine
Mirtazapine is a tetracyclic antidepressant that is used for depression, anxiety, insomnia, decreased appetite, and pain syndromes.
What is this analysis used for?
- To monitor drug concentrations in the blood.
When is the test scheduled?
- During therapy with mirtazapine (especially if liver and kidney function are impaired).
Synonyms Russian
“Remeron”, “Mirtazonal”, “Mirzaten”, “Avanza”, “Zispin”.
English synonyms
Mirtazapine (“Remeron”, “Mirtazonal”, “Mirzaten”, “Avanza”, “Zispin”).
Research method
Polarization fluoroimmunoassay (PFIA).
Units
ng/ml (nanograms per milliliter).
What biomaterial can be used for research?
Venous blood.
How to properly prepare for research?
- Do not eat for 2-3 hours before the test; you can drink clean still water.
- Do not smoke for 30 minutes before the test.
General information about the study
According to scientific hypotheses, depressive disorders occur when there is a deficiency of norepinephrine and/or serotonin in certain brain structures. In order to treat these pathologies, mood-stabilizing drugs are used - thymoleptics.
Mirtazapine is an antidepressant that can be used as a sedative, hypnotic, appetite stimulant, antiemetic and antihistamine. The drug is classified as a central agonist of alpha-2 adrenergic receptors, but its effect on serotonin, dopamine, histamine and some other receptors is also known. It is primarily used in the treatment of depression, but it is also effective for anxiety disorders, obsessive-compulsive disorder, panic attacks, post-traumatic stress disorder, anorexia, insomnia, headaches and migraines.
Mirtazapine is rapidly absorbed (bioavailability is about 50%), reaching maximum plasma concentrations in approximately 2 hours. Up to 85% of the drug binds to plasma proteins. When using 15-45 mg of mirtazapine per day, a stable plasma concentration of the substance is achieved by the fifth day of regular use. At therapeutic doses, the metabolism of mirtazapine has a linear dependence on the administered amount of the drug. Its average half-life is 20-40 hours. Sometimes, especially in older people, a longer half-life is observed (up to 65 hours), and in young people it is shorter. The long half-life of mirtazapine allows you to take the drug once a day (at night). The main pathways of its metabolism are demethylation and oxidation followed by conjugation. Some metabolites of mirtazapine are pharmacologically active and have the same pharmacological effects as the parent substance. The drug is completely eliminated approximately 4 days after cessation of administration, with 85% excreted in the urine and 15% through the gastrointestinal tract. About 4% of the drug can be excreted unchanged in the urine.
Mirtazapine has a relatively rapid onset of antidepressant effect, a slight sedative effect, reduces anxiety, improves memory and attention, has a hypnotic effect, and stimulates appetite. It can be used not only for depression, but also for withdrawal syndromes in narcology, to reduce pain in oncology, and to correct mood disorders in psychosomatic disorders. The drug is well tolerated in patients with somatic (cardiovascular, cancer, immunodeficiency) and neurological (epilepsy, Alzheimer's disease) diseases. Mirtazapine has a pronounced analgesic effect in the treatment of depression with an algic component, migraine and chronic tension-type headache and can be used in complex therapy in patients with chronic musculoskeletal pain.
Side effects from taking mirtazapine may include drowsiness, dry mouth, weight gain, increased appetite, constipation, dizziness and fatigue. In very rare cases, allergic reactions, fainting, myelodysplasia and agranulocytosis are possible. The occurrence of suicidal thoughts cannot be ruled out. There have been isolated cases of drug overdose in combination with other drugs, which resulted in death. Treatment with mirtazapine, like other antidepressants, should be carried out under strict medical supervision.
Determining the concentration of mirtazapine in the blood is not mandatory when using the drug, but it allows you to control the therapeutically effective dose of the drug in each specific case and the degree of its interaction with other drugs taken simultaneously.
What is the research used for?
- Monitoring drug concentrations in the blood;
- optimizing treatment control through dose adjustment;
- assessment of drug interactions between several drugs;
- overdose diagnosis;
- identification of violations of the drug administration regimen.
When is the study scheduled?
- When used alone with mirtazapine or in combination with other drugs (including those associated with concomitant diseases and conditions);
- when monitoring patients with impaired liver and kidney function taking mirtazapine;
- if the drug used is insufficiently effective and the issue of dose adjustment is decided;
- if the patient is suspected of non-compliance with the drug regimen;
- if an overdose of mirtazapine is suspected (lethargy, disorientation, drowsiness, hallucinations, tachycardia, hypo- or hypertension).
What do the results mean?
Reference values: 5 - 100 ng/ml.
The results of the study are analyzed by the attending physician taking into account the dose of mirtazapine, its regimen, the age and gender of the patient, the clinical picture of the disease, concomitant pathology and the presence or absence of adverse events.
What can influence the result?
- The level of mirtazapine in the blood is reduced by carbamazepine, phenytoin, and rifampicin.
- HIV protease inhibitors, azole antifungals, erythromycin, cimetidine, and nefazodone increase mirtazapine levels.
- In case of liver and/or renal failure, the concentration of the drug in the blood increases due to a violation of its metabolism and excretion.
Important Notes
- Mirtazapine at a dose of 30 mg once daily causes a slight increase in the international normalized ratio (INR) in patients treated with warfarin. In this regard, it is advisable to monitor it in case of concomitant use of warfarin with mirtazapine.
- Patients taking mirtazapine are advised to regularly monitor their blood sodium levels and liver and kidney function tests.
- It is unacceptable to adjust the dose and regimen of the drug on your own, without the participation of a doctor.
Also recommended
- Complete blood count (without leukocyte formula and ESR)
- Leukocyte formula
- Coagulogram No. 1 (prothrombin (according to Quick), INR)
- Alanine aminotransferase (ALT)
- Aspartate aminotransferase (AST)
- Total bilirubin
- Serum sodium
- Serum creatinine
- Duloxetine
- Fluoxetine
Who orders the study?
Psychiatrist, clinical pharmacologist.
Literature
- Anttila SA, Leinonen EV (2001). "A review of the pharmacological and clinical profile of mirtazapine". CNS Drug Reviews - 7(3): 249–64.
- Shams M, Hiemke C, Härtter S. Therapeutic drug monitoring of the antidepressant mirtazapine and its N-demethylated metabolite in human serum. Ther Drug Monit. 2004 Feb;26(1):78-84.
- Timmer CJ, Sitsen JM, Delbressine LP. Clinical pharmacokinetics of mirtazapine. ClinPharmacokinet 2000 Jun; 38(6):461-74.
- Medvedev V. E. Mirtazonal (mirtazapine) in psychiatric practice: forgotten and new opportunities. Review of Psychiatry and Medical Psychology named after. Bekhtereva No. 4. – 2011.
Efficacy of mirtazapine therapy in patients with depression and affective disorders
The transition of new drugs from the laboratory to everyday medical practice is always a difficult and partly contradictory process. A balanced attitude towards a medicine comes only at the stage when the doctor has a good idea of the limits of the medicine’s capabilities, based on an understanding of its advantages and disadvantages, and assigns it a stable place in his arsenal. The time it takes for a doctor to adapt to a new drug is reduced if the doctor has the opportunity to study in more detail the features of its action, using individual techniques from scientific research itself. This allows the doctor to more accurately summarize his experience and compare it with the experience of colleagues, and critically evaluate the literature data. This article is devoted to the results of one of these projects. The new drug chosen was mirtazapine, an antidepressant that is active in both the serotonergic and noradrenergic systems and selectively blocks a2 noradrenergic and 5-HT2 and 5HT3 serotonergic receptors. This neurochemical profile distinguishes mirtazapine from all currently known drugs in this group. According to the literature, mirtazapine has a powerful antidepressant effect with low severity of side effects characteristic of both tricyclic and serotonergic antidepressants [1,2].
Materials and methods
The study included 63 patients suffering from various types of affective disorder and experiencing moderate to severe depression.
The total score on the Hamilton Depression Scale (17 points) was chosen as a criterion for the severity of the condition. Severe depression was considered if this indicator was more than 25, moderate severity was more than 18, but less than 25. According to this criterion, patients were divided almost equally: with severe depression - 34 patients (average indicator on the Hamilton scale 31.6 ± 0.8 , moderate depression - 29 patients (average Hamilton scale 19.8±0.7).
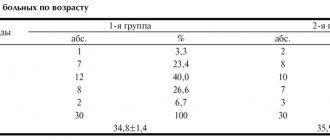
The distribution of patients by diagnostic groups, as well as the average severity of depression on an objective and subjective scale by group, is shown in Table 1.
As can be seen from the table, the greatest severity of depression was observed in patients with bipolar disorder, and the least - with chronic depressive disorder, that is, dysthymia and cyclothymia.
The research project included 12 psychiatric hospitals in Moscow, St. Petersburg and other Russian cities. There were no strict inclusion or exclusion criteria: physicians were asked to prescribe mirtazapine to all patients hospitalized for depression who were clearly eligible for antidepressant therapy. Of the antidepressants, only mirtazapine was used. Freedom was also provided in the dosage of the drug within the instructions, where the therapeutic dose varied from 15 to 45 mg. The duration of the project for each patient was 42 days.
In order to formalize the data obtained, it was proposed to use the following scales:
• Hamilton Depression Scale (17 items) [3];
• Global Clinical Impression Scale;
• Beck Self-Rating Inventory for Depression (21 items) [4].
Results and discussion
The analysis of the results was carried out in two directions: the dynamics of the condition in patients of various nosological groups and the dynamics of the condition in patients with severe and moderate depression, regardless of their nosological affiliation.
All patients, except 1 patient from the group of patients with chronic affective disorder (dysthymia), who interrupted the study due to ineffectiveness of therapy, completed the study. The percentage of responders (50% reduction on the Hamilton scale) at the end of the study, depending on the course of affective psychosis, is presented in Table 2.
Bipolar affective disorder
Statistically significant changes in the Hamilton scale were observed already by the end of the first week (p < 0.001). By the end of the study, the average total score on the scale showed the absence of depression (7.4±1.9) (Fig. 1). By the end of the first week of therapy, the most significant changes were observed in such symptoms as depressive mood, sleep disturbance and mental anxiety, while changes in the severity of depressive symptoms such as guilt, suicidal thoughts, and lethargy underwent insignificant dynamics. By the end of the study, almost all symptoms assessed by the Hamilton scale had virtually disappeared. Only the mean performance and gastrointestinal disturbance scores were 1 (the mildest degree of disturbance). According to the global clinical impression scale, this group of patients progressed from “moderate severity of the disease” (average score 4.0±0.1) through “mild severity of the disease” (average score 3.4±0.1) to “the condition borders on normal” "(average value 2.1±0.2) (p<0.001). On the self-assessment scale, statistically significant changes were also detected by the end of the first week, but were less pronounced than on the Hamilton scale (Fig. 1). By the end of the study, the Beck scale showed an even more significant improvement (p < 0.001), but still its severity remained significantly less than on the Hamilton scale.
Recurrent depression
A similar picture was observed in the group of patients with recurrent depression (Fig. 2). The difference concerned a number of symptoms that decreased in severity in the first week of therapy. The following symptoms responded most quickly to treatment by the end of the first week: depressive mood (p<0.05), suicidal thoughts (p<0.05), sleep disorder (p<0.05), gastrointestinal dysfunction (p<0.05) and weight change (p<0.01). By the end of the study, almost all symptoms had completely disappeared. The exception was the indicators of performance and depressive mood, the average values of which were still slightly greater than one, which corresponds to a mild degree of severity of the disorder.
Depressive episode
The diagnosis of “depressive episode” was given to patients if the type of course at the current stage of the disease could not be determined. In the vast majority of cases, these were patients with a very short follow-up. The general nature of the dynamics of the Hamilton, Beck and global clinical impression scales was the same as in the two previous groups of patients: statistically significant changes on all scales were detected by the end of the first week of therapy. Improvement continued to increase during treatment. However, some differences were also revealed: along with the symptom of “depressed mood” (p<0.01), the symptom of “decreased performance” (p<0.05) responded most strongly to therapy (by the end of the first week). During the same period, the weight of patients changed statistically significantly (p<0.05). That is, the primary response to therapy combined both signs of a bipolar and monopolar course. Changes in other signs of the Hamilton scale were statistically insignificant in the first period of therapy, but were almost completely reduced by the end of the study (p<0.001).
Improvement on the physician-completed scale outpaced improvement on the patient-completed scale.
Chronic mood disorder
Of the variety of chronic mood disorders, only patients with dysthymia were represented here. Unlike other groups of patients, in these patients a pronounced improvement in condition was detected later, only at the end of the study, both on an objective and subjective scale (the first week of therapy - changes in indicators on the Hamilton and Beck scale are statistically insignificant, the end of the study - p<0.001 ). Despite this, the nature of changes on the Hamilton and Beck scales practically repeated the dynamics of scale indicators in other groups (Fig. 3). According to the Hamilton scale, in the first week of therapy, a statistically significant change was observed only in relation to the symptom “decreased performance” (p<0.05). It is interesting to note that the symptom “depressive mood” was no less pronounced here than in other groups (F34 - 3.1±0.2, F33 - 3.0±0.1, F32 - 3.0±0.1, F31 – 3.3±0.2) and responded to therapy more slowly. By the end of the study, in this group there was not a single average Hamilton scale score that exceeded 1, that is, all symptoms without exception underwent reverse development.
Summarizing the above, it can be noted that, regardless of the form of affective disorder, the nature of changes in the Hamilton and Beck indices during mirtazapine therapy was the same. In all groups, the dynamics of improvement on the Hamilton scale was faster and more pronounced than on the Beck scale. In other words, the Hamilton scale turned out to be more sensitive to changes in the condition of patients than the Beck scale, which is fully consistent with the literature data [6]. However, the Beck scale also well reflected the improvement in the patients’ condition, which complemented the clinical impression. At the end of the study, there were no differences in individual symptoms recorded by the Hamilton scale. However, a significant difference was found in the set of most quickly responsive symptoms in groups with different types of disease. It is interesting that in three out of four groups the symptom “depressive mood” was statistically significantly reduced already in the first week of therapy, while in none of the groups by this time was there a reduction in feelings of guilt, a symptom that, together with a depressed mood, forms the core of depression . In two of the four groups, a rapid increase in the performance of patients was noted, and in the group of patients with dysthymia this symptom was the only one that responded to treatment in the first week; in patients with bipolar and monopolar disease, on the contrary, this symptom responded later and persisted at the end of the study.
Dynamics of the condition of patients with moderate and severe depression
Among practitioners, based on everyday clinical experience, an opinion has formed that there is a difference in the nature of the response to therapy of patients with severe and milder depression. In some cases, severe depression is associated with resistant depression. It is assumed that for the treatment of patients with such severity of the disease it is necessary to use drugs with a less selective neurochemical effect (mainly tricyclic antidepressants), doses of antidepressants should be higher, and preference should be given to intramuscular and intravenous forms. In this regard, it was especially interesting to compare the dynamics of therapeutic response rates in patients with severe and moderate depression. It was found that according to the Hamilton and Beck scales, in both groups of patients, statistically significant changes occurred already in the first week of treatment and continued to increase thereafter (Fig. 4). In the first week of therapy, changes on the Hamilton scale in the group of patients with severe depression were even slightly greater than in the group with moderate depression (the percentage of changes was 39.9% versus 37.3%, the difference did not reach the level of statistical significance). In the next therapeutic period, the dynamics were reversed: by the end of the study, the percentage of changes in the first group was 27.5%, and in the second - 35.9% (p<0.05). In other words, patients with severe depression responded more quickly to therapy, but this rate subsequently decreased, while the improvement of patients with moderate depression was more uniform. According to the Beck scale, the situation looked different: the percentage of improvement in patients with severe depression was slightly greater throughout the study than in patients with milder depression (in patients of the first group, the percentage of improvement was 17.8% in the first week and 28.9% in the second week, in patients of the second group - 14.3% and 25.3%, respectively, but the difference did not reach the level of statistical significance). That is, there was a tendency for patients with severe depression to perceive the improvement in their condition more vividly than patients with moderate depression. It should be noted that, despite the absence of restrictions on the dosage of the drug, both in the first and in the other group of patients, the doses were the same and amounted to 30 mg. Thus, in some respects, the response to therapy in patients with severe depression was even more pronounced; the oral form of the drug was not an obstacle to obtaining a high therapeutic effect in patients with severe depression; the dose of the drug did not change depending on the severity of the condition.
Side effects
Side effects were observed in a small number of patients and were mild. The most common side effects were dry mouth (6 cases), drowsiness (2 cases), hypotension (2 cases). In isolated cases, the following were observed: a feeling of weakness, insomnia, anxiety and inversion of affect. Increased appetite and weight gain, known to be the most common side effects of mirtazapine therapy (7), were not listed as side effects in this study, although the Hamilton scale items showed changes in these symptoms.
Conclusion
Analysis of the results of the study allows us to draw two blocks of conclusions. The first concerns the technique of conducting this unusual study, the second concerns its results.
1. Results based on data obtained by practitioners rather than researchers are essentially the same as results based on data obtained under the special conditions of controlled studies:
• Not only the objective Hamilton scale, but also the Beck self-rating scale were sensitive to changes that occurred during mirtazapine therapy.
• The Beck Scale has been successfully used in patients with severe depression, although according to some authors, the severity of depression limits the use of this tool [4].
2. The study showed that mirtazapine was an effective antidepressant for various forms of mood disorder:
• the general nature of the dynamics of the therapeutic response was similar in patients with different types of course, but the immediate response (the first week of therapy) was significantly different, revealing the most “sensitive” signs to mirtazapine in patients with different types of affective disorder.
• Mirtazapine was found to be equally effective as an antidepressant for severe and moderate depression (5).
• greater severity of depressive disorders did not require an increase in dose.
The list of references can be found on the website https://www.rmj.ru
Mirtazapine –
Remeron (trade name)
(Organon)
Literature
1. Mosolov S.N., Kostyukova E.G., Serditov O.V. et al. Clinical efficacy and tolerability of the new antidepressant mirtazapine (Remeron) in severe depression. Social and Clinical Psychiatry, 1, 2000, pp. 55-60.
2. Kolyutskaya E.V., Yastrebov D.V. Use of mirtazapine in the treatment of depression. Russian medical journal N19, vol. 2, N2-3, 1999, pp. 50-52
3. Hamilton M. Development of a rating scale for primary depressive illness. Brit.J, of Soc.Clin.Psychol., 1967; 6: 278-296.
4. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry 1961; 4: 561-571
5. Gorman JM. Mirtazapine: clinical overview Journ Clin Psychiatry 1999;60 (suppl 17),pp9-13
6. Moller HJ, von Zerssen D. Self-ration procedures in the evaluation of antidepressants. Psychopathology 1995; 28: 291-306
7. Guelfi JD, Ansseau M, Timmerman L et al. Efficacy and tolerability of mirtazapine vs venlafaxine in hospitalized, severely depressed patients with melancholia. Abstracts ECNP, Brussels, 2000.
| Applications to the article |
| Rice. 1. Dynamics of the general indicator according to the Hamilton and Beck scales (bipolar current) |
| Rice. 2. Dynamics of the general indicator according to the Hamilton and Beck scales (unipolar depressive course) |
| Rice. 3. Dynamics of the general indicator according to the Hamilton and Beck scales (dysthymia) |
| Rice. 4. Dynamics of the general indicator according to the Hamilton and Beck scales (moderate and severe depression) |
Drinking alcohol while being treated with antidepressants - is there a reason for concern?
Translation by neurologist M.A. Yablonsky
Drinking alcohol during antidepressant treatment — a cause for concern?
| News | Pharmaceutical Journal
. (n.d.).
Retrieved February 1, 2021,
New evidence indicates a risk of pathological intoxication when patients receiving SSRI treatment drink alcohol. Andrew Herxheimer and David B. Menkes investigate
Preclinical studies of the interaction between selective serotonin reuptake inhibitors (SSRIs) and alcohol have primarily been conducted in acute studies in healthy volunteers using various assessments of psychophysiological function.
Most often, simultaneous use does not lead to functional impairment and does not cause effects different from those of alcohol.1,2
Alcohol use is common in depression,3 but its interaction with antidepressants in patients (excluding alcohol abusers) has not been well studied. A subgroup of people who drink alcohol are likely to experience greater difficulties.4,5
In our practice, we have repeatedly noted that some people experience pronounced changes in alcohol tolerance during treatment with SSRIs and related drugs. Consequences include sexual disinhibition and increased episodes of violence.6,7 This pattern of changes is common with SSRIs and venlafaxine,8 but is often not given sufficient attention by clinicians. The mechanism of this phenomenon is not well understood. It is hypothesized that the disinhibitory effect of alcohol, together with the stimulant effect of most SSRIs and related antidepressants, may produce effects that are not seen with the substances alone. Given this problem, we are reviewing the warnings for patients and prescribers in the prescribing information for SSRIs and related drugs provided by the manufacturer.
Pathological intoxication
Pathological intoxication, based on anecdotal reports of the use of normal amounts of alcohol, resulting in either significant intense intoxication or qualitatively different intoxication, such as highly unusual disinhibition or aggression
Literature Search
We reviewed the drug's generic clinical pharmacology (TCPS) and patient information leaflets (PIS) and identified all references to interactions with alcohol, including warnings for venlafaxine, mirtazapine, bupropion, duloxetine and each of the SSRIs available on the UK market.
In 2006–07, we surveyed chief medical officers of major pharmaceutical companies receiving any information about possible interactions of their drugs with alcohol, including pilot studies with volunteer participants, clinical studies, and case reports of possible interactions.
Table 1 provides information on the TKFS and ILP. Almost all information leaflets for clinicians and patients mentioned in some form the need to avoid drinking alcohol while taking drugs (SSRIs or venlafaxine) or to do so with caution (duloxetine). In most of them, this recommendation is not substantiated in any way. In some, this point of view is generally accepted. For example, the TCPS for fluvoxamine states: “As with other psychotropic medications, patients should not drink alcohol.”
Table 1 Warnings in the Summary of Product Characteristics (SPC) or Patient Information Leaflet (PIS) (November 2011, www.medicines.org/emc)
| Citalopram Cipramil Lundbeck | No pharmacokinetic or pharmacodynamic interaction was detected between citalopram and alcohol. However, simultaneous use of citalopram and alcohol is not recommended. | As with other antidepressants, it is advisable to avoid alcohol during treatment, although Cipramil has not been shown to increase the effects of alcohol. |
| Escitalopram Cipralex Lundbeck | No pharmacokinetic or pharmacodynamic interaction is expected between escitalopram and alcohol. However, as for other psychotropic drugs, their combination with alcohol is not recommended. | As with many medications, the combination of Cipralex with alcohol is not recommended, and interaction of Cipralex with alcohol is not expected. |
| Duloxetine Cymbalta Lilly | Medicinal products that act on the central nervous system The risk of administering duloxetine in combination with other drugs that act on the central nervous system has not been systematically evaluated except as described in this section. Therefore, special care is recommended when prescribing Cymbalta in combination with other drugs or substances that act on the central nervous system, including alcohol and sedatives (for example, benzodiazepines, morphinomimetics, antipsychotics, phenobarbital, sedating antihistamines). | Cymbalta can be taken either with food or on an empty stomach. Caution is necessary when drinking alcohol during treatment with Cymbalta. |
| Fluoxetine Prozac Lilly | Alcohol By standard assessment, fluoxetine did not increase blood alcohol levels or enhance the effects of alcohol. However, simultaneous use of SSRIs and alcohol is not recommended. | You can take Prozac with or without food. You should avoid drinking alcohol while you are being treated with this drug. |
| Fluvoxamine. Fevarin Abbott | As with other psychotropic medications, patients are advised not to drink alcohol while taking fluvoxamine. | Taking Fevarin with food or drinks Do not drink alcohol if you are taking this drug. This is due to the fact that the effect of alcohol when taking Fevarin causes drowsiness and instability. |
| Mirtazapine Zispin MSD | Mirtazapine may enhance the depressant effect of alcohol on the central nervous system. Patients should be advised to avoid alcoholic beverages during treatment with mitrazapine. | Taking Mirtazapine with food and alcoholic beverages. You may feel drowsy if you drink alcohol at the same time as taking Mirtazapine. It is recommended not to consume any alcoholic beverages. |
| Paroxetine Seroxat GSK | Alcohol As with other psychotropic drugs, it is advisable to avoid drinking alcohol while taking paroxetine. Clinical experience shows that treatment with paroxetine is not accompanied by impairment of cognitive or psychomotor functions. However, as with all psychoactive drugs, patients must be careful when driving and operating machinery. Although paroxetine does not increase alcohol-induced impairment of cognitive and motor skills, concomitant use of paroxetine and alcohol is not recommended. | Do not drink alcohol at the same time as taking Paroxetine. Alcohol may worsen your symptoms or side effects. |
| Sertraline Lustral Abbott [formerly Pfizer] | CNS depressants and alcohol Co-administration of sertraline 200 mg daily does not enhance the effects of alcohol, carbamazepine, haloperidol or phenytoin on cognitive or psychomotor functions in healthy individuals. However, simultaneous use of alcohol and sertraline is not recommended. | While taking Sertraline, you should avoid drinking alcohol. |
| Venlafaxine Efexor XL Wyeth | When taking venlafaxine, it was shown that there was no increase in motor and cognitive impairment caused by ethanol. However, as with other drugs that act on the central nervous system, patients are advised to avoid alcohol. | It is recommended to refrain from drinking alcohol while taking Venlafaxine. |
But the relevant SLP makes a clearer statement: “alcohol may interfere with the effect of the drug by causing drowsiness and unsteadiness.” Similarly, the GSK PMI states that taking alcohol concomitantly with paroxetine may increase symptoms or the severity of adverse events. Five of the six UK companies that market their products (GSK, Lilly, Lundbeck, Pfizer and Wyeth) cite experimental data indicating that their drug(s) do not increase alcohol-related adverse events, but still do not recommend use alcohol without convincing explanation.
Companies' responses to our follow-up requests for alcohol interaction data varied widely (Table 2). Some came from medical directors and medical advisers, others from pharmacovigilance staff. Everyone was polite, but none of them expressed interest. In most cases, summaries of one or more experimental studies involving healthy volunteers were sent, indicating that concomitant single administration of drugs with alcohol did not cause more severe mental or motor impairment than alcohol alone. Several submitted reports or summaries of published or unpublished clinical studies in alcoholics assessing the drug's ability to reduce alcohol consumption or prevent relapse into alcoholism. None of them showed a positive effect.
One company (GSK) submitted an impressive amount of literature data, however, the collection included a large number of results from animal studies of questionable significance. No case reports were submitted by any of the companies; most referred only to the number of cases in the UK Medicines and Healthcare Products Agency's Yellow Card Register. One company stated that the text of these messages was confidential. None of the international companies cited case reports from outside the United States.
To drink or not to drink?
We identified a general trend in TCPS and ILPs prepared by manufacturers of SSRIs and other antidepressants. Despite all the problems with alcohol use, some mixed reports cited data from studies involving healthy volunteers and indicated that the drug did not interact with alcohol.
Table 2 Company reports for 2006-07
| Company | A drug | Who reported | Message content | Sent documents | Reports of adverse reactions to the drug |
| Lundbeck | Citalopram, Escitalopram | Senior Pharmacovigilance Specialist | Four-page letter, review, eight references to published studies | Eight articles and abstracts included, some of them on alcohol abusers | An analysis of 13 cases is provided |
| Lilly | Fluoxetine | Principal Health Information Researcher | Brief characteristics of the drug | Taking Prozac with alcohol: four-page review, 18 links | Number of requests for descriptions of our cases |
| Duloxetine | Principal Health Information Researcher | Brief characteristics of the drug | Use in patients who abuse alcohol | ||
| GSK | Paroxetine | Head of Medical Department | Review of three published and three unpublished research results | Also 47 references to studies on the interaction of alcohol with other SSRIs. Subsequently, unpublished results of a study of paroxetine for the prevention of relapse in alcoholism were sent upon request. | Number of requests for descriptions of our cases |
| Wyeth | Venlafaxine | Scientific consultant specializing in CNS pathology | Narrative review, two pages, 4 references | There were fewer than 10 reported interactions in the UK | |
| Pfizer | Sertraline | Medical Spokesperson | 11 healthy participants received 200 mg for nine days, then one dose of alcohol at a rate of 0.5 g/kg. No effect on cognitive or psychomotor functions noted | Unpublished experiment | Four reports to the Medicines and Healthcare Products Regulatory Agency - yellow cards |
Warnings regarding alcohol intake are therefore not supported by relevant evidence. They appear unconvincing to both patients and prescribers. This may explain why many patients do not take such warnings seriously.
We have described a syndrome of pathological intoxication, often with serious consequences, in patients prescribed SSRIs or related drugs.6–8
It is noteworthy that only small or ordinary amounts of alcohol are often involved, and that memory impairment is noted in more than half of the cases. This problem is not rare, but often it does not receive enough attention. This may be due to the known underestimation of adverse events and the possibility that regulators are not systematically assessing such effects across drug classes. For example, the MHRA online database contains 129 reports labeled “alcohol interactions” with SSRIs or related drugs, but these were not combined into a specific group. Messages that were combined by drug name and drug association were not identified.9
It is necessary to supplement the TKFS and ILP with an unambiguous warning to doctors and patients about the possibility of developing pathological alcohol intoxication during treatment with SSRIs and similar drugs. This should help in choosing medications, identifying and studying this problem.
List of used literature
- Allen D, Lader M. Interactions of alcohol with amitriptyline, fluoxetine and placebo in normal subjects. International Clinical Psychopharmacology 1989;4(Suppl 1):7–14.
- Schaffler K. Study on performance and alcohol interaction with the antidepressant fluoxetine. International Clinical Psychopharmacology 1989;4(Suppl 1):15–20.
- Davis LL, Rush JA, Wisniewski SR, Rice K, Cassano P, Jewell ME et al. Substance use disorder comorbidity in major depressive disorder: an exploratory analysis of the Sequenced Treatment Alternatives to Relieve Depression cohort. Comprehensive Psychiatry 2005;46:81–9.
- Chick J, Aschauer H, Hornik K. Efficacy of fluvoxamine in preventing relapse in alcohol dependence: a one-year, double-blind, placebo-controlled multicentre study with analysis by typology. Drug and alcohol dependency 2004;74:61–70.
- Lingford-Hughes AR, Welch S, Nutt DJ. Evidence-based guidelines for the pharmacological management of substance misuse, addiction and comorbidity: recommendations from the British Association for Psychopharmacology. Journal of Psychopharmacology 2004;18:293–335.
- Chandler P, Herxheimer A. Unexpected aggressive behavior: interaction of bupropion and alcohol. International Journal of Risk and Safety in Medicine 2011;23:133–7.
- Herxheimer A. British diplomat cleared of drunk flying charges: paroxetine was involved. International Journal of Risk and Safety in Medicine 2007;19:35–40.
- Menkes DB, Herxheimer A. Provocation by alcohol of violence as a side-effect of antidepressants. Drug Safety 2009;32:948–9.
- Download Drug Analysis Prints (DAPs). London: Medicines and Healthcare products Regulatory Agency (MHRA); 2011. Available at: www.mhra.gov.uk (accessed 30 November 2011).
Use of the drug Mirtazapine
The starting dose for adults is 15 mg/day. The average effective therapeutic dose is 15–45 mg. When the maximum daily dose is reached and there is no clinical effect over the next 2-4 weeks, treatment should be discontinued. Therapy is continued until clinical symptoms of depression disappear completely within 4–6 months. Cancelled gradually. In elderly patients, the dose of mirtazapine is increased gradually and under strict medical supervision. There is no data on the safety and effectiveness of mirtazapine in children.
Pharmacological properties of the drug Mirtazapine
Antidepressant. It blocks central presynaptic α2 receptors and also enhances nerve transmission at serotonergic synapses only through 5-HT1 receptors, since 5-HT2 and 5-HT3 receptors are blocked. Reduces the severity of symptoms such as anhedonia, psychomotor retardation, sleep disturbances, weight loss, suicidal thoughts, mood swings, loss of interest in life. The antidepressant effect develops after 1–2 weeks of treatment. Mirtazapine after oral administration is rapidly absorbed from the digestive tract. Bioavailability - 50%. The maximum concentration in the blood plasma is achieved after 2 hours. The equilibrium concentration of mirtazapine in the blood plasma is established after 3-4 days of continuous use. Plasma protein binding is 85%. Mirtazapine is extensively metabolized in the liver by demethylation and oxidation followed by conjugation. Dimethylmirtazapine is as pharmacologically active as the parent substance. Mirtazapine is excreted in urine and feces. The half-life is 20–40 hours.
Among the antidepressants currently used both abroad and in our country, milnacipran remains the least studied. Thus, at the time of writing the review, the Internet portal of the National Library of Medicine of the US National Institutes of Health contains 579 links to publications that mention this drug (Table 1). If we take into account that the first publication appeared in 1985 [1], then on average over the past 30 years, only about 19 published works were published annually, one way or another related to milnacipran. This is the lowest rate among all antidepressants currently used both abroad and in our country. That is why there is a need to devote a special review of the literature to milnacipran and its role in the treatment of various disorders.
Table 1. Data on links to publications mentioning antidepressants on the Internet portal of the National Library of Medicine of the US National Institutes of Health Note. NASSA - noradrenergic and specific serotonin antidepressants; CCA - four-cyclic antidepressants; ASIOZ - serotonin antagonists and serotonin reuptake inhibitors.
It is known that this drug was originally called midalcipran [1]. After renaming, it was registered in European countries in 1997 for the treatment of depressive conditions of varying severity [2]. Their criteria are given in table. 2, taking into account the main and additional symptoms [3]. The main ones are represented by deterioration in mood, loss of interests and pleasure, and decreased energy, which can lead to increased fatigue and impaired activity. Additional ones are characterized by decreased ability to concentrate and pay attention, low self-esteem and feelings of self-doubt, ideas of guilt and humiliation, a gloomy and pessimistic view of the future, thoughts or actions aimed at self-harm or suicide, as well as sleep disturbances and decreased appetite. In addition, somatic symptoms are included in a special category. These include: loss of interests and pleasure in activities that were previously enjoyed, loss of emotional response to the environment and events that are usually pleasant. Waking up in the morning 2 hours (or more) earlier than usual, objective (noted by a stranger) signs of psychomotor retardation or agitation are also typical; severe decrease in appetite or libido; weight loss of 5% (or more) over the past month, as well as greater severity of depression in the morning. Four or more of these symptoms form a somatic syndrome. The latter, along with psychotic symptoms (delusions and hallucinations), is preferred for a major depressive episode.
Table 2. Criteria for the severity of a depressive episode according to ICD-10 Note. * - for acute and severe symptoms.
It is believed that a depressive episode may be the only manifestation of a mental disorder (recurrent depressive disorder, dysthymia) or interspersed with other syndromes (eg, manic, as in bipolar affective disorder, or cyclothymia). But besides this, depressive episodes (usually mild to moderate severity) can be observed against the background of neurological and somatic diseases.
The appearance of milnacipran on the pharmaceutical market was met with enthusiasm [4]. The fact is that by that time, selective serotonin reuptake inhibitors (SSRIs) began to be increasingly used in European psychiatry, which, unlike tricyclic antidepressants (TCAs), “lost not only their direct effect on the receptors responsible for adverse side effects, but also the ability to inhibit the reuptake of norepinephrine" [4], necessary for a therapeutic effect. As a result, new antidepressants have become much better tolerated by patients. But SSRIs “tend to be less effective than TCAs, especially for more severe forms of depression” [4].
It was assumed that the “supernova” selective serotonin and norepinephrine reuptake inhibitors (SNRIs) would have a stronger antidepressant effect than SSRIs, and would be comparable in tolerability [5]. Accordingly, SNRIs, and especially one of the representatives of this group, milnacipran, can replace TCAs even in the treatment of severe depression. Such hopes were based on the fact that this antidepressant almost equally inhibits the reuptake of both serotonin and norepinephrine. while other SNRIs (venlafaxine and duloxetine) have a greater effect on the metabolism of the first of these two neurotransmitters. Many authors sought experimental confirmation of this hypothesis [6, 7]. Unfortunately, the research results did not coincide with expectations. In particular, it was reported [8] that the results of clinical studies did not establish differences in the effectiveness of SSRIs and milnacipran, and its tolerability is similar to fluoxetine and fluvoxamine. Thus, TCAs remained first-line drugs, at least for severe depression. Finally, it was noted [8] that the considered representative of the SNRI group, although it gives less anticholinergic effects (sedation and sweating) than TCAs, but, like them, can cause dysuria [8].
Publications on the topic under consideration were summarized in the form of a meta-analysis of the Cochrane Database [9]. It stated that there was insufficient evidence to conclude that milnacipran is superior, inferior, or equal to other antidepressants in effectiveness and tolerability in the acute phase of treatment of major depression. If there is some evidence to support the superiority of milnacipran over TCAs, it concerns the proportion of patients who dropped out of the study due to adverse events and some tolerability parameters. Finally, after 12 years of clinical use, the authors of the meta-analysis recommended further studies to answer the question of whether milnacipran would be a better antidepressant for the treatment of acute major depression.
These recommendations were not implemented, in part because information about the drug's mechanism of action was revised. Milnacipran turned out to be a “more noradrenergic” rather than a “balanced serotonin and norepinephrine” drug [10]1. In addition, new ideas have emerged about the pathogenesis of depression itself.
In the neurochemical aspect, symptoms of depression can be differentiated into two main clusters: norepinephrine and serotonin-norepinephrine [13]. The first of them is associated with a deficiency in the activity of norepinephrine neurons and is manifested by symptoms such as psychomotor retardation, loss of concentration, energy, fatigue, and tiredness. The second cluster is caused by a decrease in the activity of serotonin and norepinephrine neurons. It is characterized by a depressed mood, loss of interests or the ability to feel pleasure, sleep disturbances, feelings of uselessness, pessimism and anxiety. In light of such data, it may be possible to explain why milnacipran has not emerged as a superior antidepressant for the treatment of acute severe depression. It turned out that his therapeutic activity was addressed not so much to the key manifestation of depression - low mood, but to symptoms less specific to a depressive state (psychomotor retardation, loss of concentration, lack of energy, fatigue, tiredness). But the same feature of the mechanism of action of milnacipran has made it possible to recommend it for the treatment of patients with disorders of social adaptation, which are observed both in the structure of depression itself and its residual symptoms. Such recommendations were clearly not promising for the clinical use of milnacipran in depression. Therefore, in the future, most publications turned out to be related to the use of an antidepressant for fibromyalgia (this indication was assigned to milnacipran in the USA in 2009).
Fibromyalgia is a rheumatological disease whose diagnostic criteria have changed repeatedly over its 110-year history [14]. For example, 5 years ago, sensitive trigger points located on certain muscles of the body were used to identify this pathology. However, in 2010, these points were abandoned and the possibility of establishing this diagnosis was extended to patients with less intense pain. At the same time, the criteria were supplemented by various somatic symptoms and cognitive impairment (“fibrofog” in the head).
As a result, the diagnostic criteria for fibromyalgia took on the following form [15]. The patient must have 3 signs: 1) pain index (PI) >7 and total score on the symptom severity scale (TSS) >5 or BI 3-6 and TSS >9; 2) symptoms must be present at the same level for at least 3 months; 3) the patient should not have another disorder that could explain the pain.
To calculate the BI, it is determined whether the patient has experienced pain in 14 symmetrical (left and right: shoulder girdle, upper and lower arms, hip/buttock and greater trochanter, upper and lower legs, jaw) and 5 asymmetrical (chest) over the last week , abdomen, upper and lower back, neck) areas of the body. Pain in each of these areas is scored 1 point. Subsequently, the points are summed up. Accordingly, the total BI score can range from 0 (minimum) to 19 (maximum).
The total PTS score includes an assessment of the severity of 3 main (excluding pain) symptoms (fatigue, sleep disturbances, cognitive symptoms), as well as the severity of 40 additional symptoms (myalgia, irritable bowel syndrome, fatigue/fatigue, thinking or memory disorders, muscle weakness, headache, abdominal pain/cramping, numbness/tingling, dizziness, sleep disturbance, depression, constipation, lower abdominal pain, nausea, nervousness, chest pain, blurred vision, fever, dry mouth, itching, noisy breathing, phenomenon Raynaud's, hives, tinnitus, vomiting, heartburn, oral ulcers, loss/change in taste, seizures, dry eyes, shortness of breath, loss of appetite, rash, sensitivity to light, hearing loss, bruising easily, hair loss, increased urination, painful urination, spastic bleeding). Each of the 3 main signs must be assessed by the patient on a scale: 0 points (no violations); 1 point (mild and minor impairments, usually subtle or transient); 2 points (moderate and significant disturbances, appearing many times, and/or constant moderate severity); 3 points (severe violations, permanent, long-term, life-threatening). As a result, a patient can score from 0 to 9 points in this section of the STS. To determine the severity of 40 symptoms, another scale is proposed: 0 points (no symptoms); 1 point (few symptoms - from 1 to 5); 2 points (moderate number from 5 to 10 symptoms); 3 points (large number - more than 10 symptoms). Accordingly, a patient can score from 0 to 3 points in this section of the STS. Thus, the final score on the ShTS ranges from 0 to 12 points.
As for milnacipran, the possibility of its use in fibromyalgia was justified using the concept of increasing the sensitivity of the central nervous system to pain and impairing its modulation [16]. It is known that two key neurotransmitters in pain pathways are serotonin and norepinephrine. TCAs that inhibit the reuptake of serotonin and norepinephrine and increase their concentrations in the central nervous system are effective in the treatment of fibromyalgia. However, long-term use of TCAs is limited due to problems with poor tolerability. This is why new SNRIs, particularly milnacipran, have been recommended for the treatment of fibromyalgia [17]. The overall effect of milnacipran was assessed as significant but moderate [18]. At a daily dose of 100 mg or 200 mg, it reduced the severity of pain by 30% or more in 40% of patients with fibromyalgia [19]. Cases have been identified where treatment of fibromyalgia with milnacipran is preferable. In particular, an antidepressant should be used if the clinical picture of the disease shows pronounced symptoms of the norepinephrine cluster mentioned above: loss of energy, fatigue, tiredness, accompanied by limitation of daily activity [20, 21].
It was also found that milnacipram should be prescribed for complaints of intense pain [20]. Moreover, the effectiveness of milnacipran in this situation turned out to be associated with new, previously unknown aspects of the drug’s mechanism of action. The latter blocks NMDA receptors located on glutmate neurons of the dorsal horns of the spinal cord, which are involved in pain amplification processes [22, 23]. One such process is the so-called winding-up phenomenon—a progressive increase in pain sensations in response to repeated and rhythmic stimuli of equal intensity reaching sensory glutamate neurons. These neurons, in turn, activate nearby ones, which is manifested by an expansion of the area of hyperalgesia with its spread to areas of the skin innervated by neighboring nerves (the phenomenon of secondary hyperalgesia). In addition, an increase in the excitability of dorsal horn neurons leads to a decrease in the pain threshold [24]. In this case, painful sensations appear in response even to tactile irritation, which is normally not accompanied by any complaints at all (the phenomenon of allodynia).
The presented data on the mechanism of action of milnacipran in fibromyalgia have led to renewed interest in the problem of using this antidepressant in patients with depression. The fact is that glutamate neurons are involved not only in the regulation of processes associated with the perception of pain. Another function of these neurons, located not in the spinal cord, but in the brain, is inhibition of dopamine nerve cells [25]. Accordingly, blockade of NMDA receptors by milnacipran will be accompanied by a double effect. On the one hand, a decrease in the activity of glutamate neurons, and on the other, a disinhibition of dopamine neurons. As a result, milnacipran, while not being a dopamine reuptake inhibitor [26], is capable (albeit indirectly) of activating the dopamine system, which has been repeatedly noted in relevant experimental studies [27, 28].
The combination of milnacipran's ability to act on norepinephrine and dopamine neurochemical systems determines its effectiveness in one of the three main types of low mood that are observed in depression (Table 3) - with a decrease in the activity of dopamine and norepinephrine neurons. This option is characterized by a weakening of positive emotions: depression, joylessness, loss of interests and desires, hopelessness, uncertainty. In addition, milnacipran, as mentioned earlier, can also be used in the presence of symptoms of depression, indicating a decrease in the activity of norepinephrine neurons (psychomotor retardation, loss of concentration, energy, fatigue, tiredness).
Table 3. Main variants of low mood in depression [29] Note. YES - dopamine, NA - norepinephrine, SE - serotonin.
Thus, milnacipran is indicated for the pharmacotherapy of depression, the pathogenesis of which involves dopamine and norepinephrine neurochemical systems. Clinically, we are talking about apathetic, anhedonic, adynamic and asthenic depression. Taking into account the fact that all of them are potential targets for milnacipran, it is adequate to combine them into one type of depression, based on the presence of the letter “a” in the name: accordingly, apathetic, anhedonic, adynamic and asthenic depressive states can be designated as “depression A- kind of."
From the table Figure 4 shows that not all antidepressants are able to activate dopamine and norepinephrine neurons. In particular, most drugs do not have this effect - clomipramine, venlafaxine (in medium and low daily doses), duloxetine, mirtazapine, maprotiline, mianserine, sertraline, citalopram, escitalopram, paroxetine, fluoxetine, fluvoxamine. Accordingly, the mechanism of action of the listed antidepressants is inadequate to the features of the pathogenesis of type A depression.
Table 4. Main effects and mechanisms of action of antidepressants [29] Note. * - venlafaxine in high doses (>225 mg/day), ** - venlafaxine in medium doses (150-225 mg/day), *** - venlafaxine in low doses (<150 mg/day), **** - neurons: DA - dopamine, NA - norepinephrine, C - serotonin, HI - histamine, AC - acetylcholine.
But even with the ability to activate dopamine and norepinephrine neurons, the pharmacological properties of an antidepressant may include a number of “inconvenient” moments (see Table 4). Thus, amitriptyline, imipramine and venlafaxine increase the activity of serotonin neurons. Meanwhile, they are able to inhibit (albeit moderately) the function of norepinephrine and dopamine neurons [25]. But the more effectively the listed antidepressants act on serotonin metabolism, the weaker their effect on norepinephrine and dopamine in the central nervous system. Accordingly, this mechanism of action is also not entirely suitable for the already mentioned features of the pathogenesis of type A depression. In addition, amitriptyline, imipramine and trazodone inhibit norepinephrine, histamine and acetylcholine neurons (see Table 4), having sedative properties. Meanwhile, with type A depression, motor and ideational retardation, fatigue, weakness, etc. are often observed. It is easy to imagine how in this situation the sedative effect of the drug will contribute to the aggravation of these complaints, and therefore will complicate the implementation of pharmacotherapy.
Finally, when selecting an antidepressant based on its mechanism of action, one should consider how exactly it activates norepinephrine and dopamine neurons. For example, agomelatine and trazodone do this by influencing receptors. However, this mechanism of action is not the most effective. It is believed that a direct effect on monoamine metabolism contributes to greater pharmacological activity of the antidepressant [29].
From the above it follows that the mechanism of action of milnacipran is best suited to the characteristics of the pathogenesis of type A depression. So this drug (unlike clomipramine, venlafaxine in medium and low daily doses, duloxetine, mirtazapine, maprotiline, mianserine, sertraline, citalopram, escitalopram, paroxetine, fluoxetine, fluvoxamine) increases the activity of dopamine and noradernaline neurons. Milnacipran (unlike amitriptyline, imiparmine and venlafaxine) is not capable of pronounced activation of serotonin neurons, but its effect on dopamine and norepinephrine nerve cells in this case may be more complete. Milnacipran (unlike amitriptyline, imipramine and trazodone) does not inhibit norepinephrine, histamine and acetylcholine neurons and does not have sedative properties. In this regard, it should be better tolerated by patients. Finally, milnacipran (unlike agomelatine and trazodone) affects norepinephrine neurons through its effect on their metabolism, and not through receptors. In other words, a more efficient mechanism of action is used.
The presented data on the pharmacological properties of milnacipran allow us to consider it as a first-choice drug in the treatment of type A depression. But this must be confirmed in special studies performed according to a single protocol in several certified centers using methods of randomization of observations and double-blind monitoring of results.
Concluding this review of the literature, we should dwell on the prospects for the use of milnacipran in our country. The only indication for this antidepressant registered in Russia is depressive disorders of varying severity3. Obviously, this formulation allows the widespread use of milnacipran in the treatment of type A depression, the prevalence of which, unfortunately, has not yet been studied. However, according to some data, they are often found in both affective disorders (including bipolar) and schizophrenia [29]. In addition, type A depression is often encountered in neurological practice in patients with chronic cerebral ischemia [30] and Alzheimer’s disease [31]. At the same time, in our country there is no second indication for the use of milnacipran for fibromyalgia. As mentioned above, this is due not so much to doubts about the effectiveness of the antidepressant, but to the attitude towards the diagnosis itself, which is actually not used in clinical practice. Some authors [32] suggest that this is due to the unusual manifestations of the disease and the lack of clear diagnostic criteria. But other explanations for the current situation are also possible.
Fibromyalgia is considered to be clinically indistinguishable from neurasthenia [14]. In any case, the diagnostic criteria for these disorders are similar. To verify this, just look at the main signs of neurasthenia in ICD-10. Thus, this disorder is characterized by constant complaints of increased fatigue after mental work or weakness in the body and exhaustion after minimal effort, as well as at least two symptoms from the list (feeling muscle pain, dizziness, tension headaches, sleep disturbances, inability to relax, irritability, dyspepsia). Based on these signs, fibromyalgia in Russia may well be diagnosed as neurasthenia. This diagnosis in our country, according to E.V. Kalinina [33] is established in 9% of patients who first sought psychiatric help. Moreover, it is emphasized that more than 5-6 years usually pass between the onset of the disease and the first consultation with a psychiatrist, when patients are observed by therapists, neurologists and other specialists.
In the presented situation, one should not count on the rapid development of new indications for the use of milnacipran, taking into account the possibility of its use in fibromyalgia. In this regard, a sufficiently large number of patients will not be able to receive effective therapy with milnacipran. The only exception can be those patients whose clinical picture of fibromyalgia is accompanied by depression.
The reasons for the differences in information about the mechanism of action of milnacipran are an independent problem and are not analyzed in this review. Let us only point out that they are associated with a variety of experimental techniques and possibilities for selecting nervous tissue materials [11, 12].
This effect level is considered clinically significant.
Instructions for the medical use of the drug Ixel https://grls.rosminzdrav.ru/InstrImg.aspx?idReg=21215&t=&isOld=1
Special instructions for the use of the drug Mirtazapine
The results of experimental studies indicate the absence of a teratogenic effect, however, there are no clinical data on the effectiveness and safety of mirtazapine during pregnancy and lactation. The drug is prescribed with caution to patients with epilepsy and organic brain lesions, with impaired liver and/or kidney function, patients with angina pectoris and/or recent myocardial infarction, with cardiac conduction disorders, with arterial hypotension, with urinary disorders due to prostate hypertrophy, patients with acute angle-closure glaucoma and increased intraocular pressure, with diabetes mellitus. When using mirtazapine in patients with schizophrenia or other psychotic disorders, psychotic symptoms and delusional experiences may increase. When treating the depressive phase of biopolar affective disorders, this condition can progress to the manic phase. It should be borne in mind that if symptoms such as fever, sore throat, stomatitis develop while taking mirtazapine, treatment should be stopped immediately. Abrupt withdrawal of mirtazapine after long-term use can lead to nausea, headache and a general deterioration in health. During treatment with mirtazapine, you should refrain from drinking alcohol. Mirtazapine should be prescribed with caution to patients engaged in potentially hazardous activities that require increased attention and speed of psychomotor reactions (including driving).