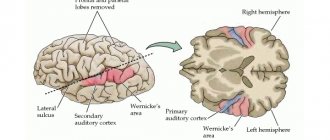
The role of the cerebral cortex
In the posterior central gyrus, behind the central sulcus, there is an area of cutaneous and joint-muscular sensitivity.
Here the signals that arise when touching our body, when it is exposed to cold or heat, or when it is painful are perceived and analyzed. In contrast to this zone, in the anterior central gyrus, in front of the central sulcus, the motor zone is located. It identifies areas that provide movement of the lower extremities, muscles of the trunk, arms, and head. When this area is irritated by electric current, contractions of the corresponding muscle groups occur. Injuries or other damage to the motor cortex lead to paralysis of the body muscles.
The auditory area is located in the temporal lobe. The impulses arising in the receptors of the cochlea of the inner ear are received here and analyzed. Irritation of areas of the auditory zone causes sensations of sounds, and when they are affected by the disease, hearing is lost.
The visual zone is located in the cortex of the occipital lobes of the hemispheres. When it is irritated by electric current during brain surgery, a person experiences sensations of flashes of light and darkness. When it is affected by any disease, vision deteriorates and is lost.
Near the lateral sulcus there is a taste zone where taste sensations are analyzed and formed based on signals arising in the receptors of the tongue. The olfactory zone is located in the so-called olfactory brain, at the base of the hemispheres. When these areas are irritated during surgery or during inflammation, people smell or taste something.
There is no purely speech zone. It is represented in the cortex of the temporal lobe, the inferior frontal gyrus on the left, and parts of the parietal lobe. Their diseases are accompanied by speech disorders.
The cerebral hemispheres occupy about 80% of the volume of the cranium, and consist of white matter, the basis of which consists of long myelinated axons of neurons. The outside of the hemisphere is covered by gray matter or the cerebral cortex, consisting of neurons, unmyelinated fibers and glial cells, which are also contained in the thickness of the sections of this organ.
The surface of the hemispheres is conventionally divided into several zones, the functionality of which is to control the body at the level of reflexes and instincts. It also contains the centers of higher mental activity of a person, ensuring consciousness, assimilation of received information, allowing adaptation in the environment, and through it, at the subconscious level, through the hypothalamus, the autonomic nervous system (ANS) is controlled, which controls the organs of circulation, respiration, digestion, excretion , reproduction, and metabolism.
In order to understand what the cerebral cortex is and how its work is carried out, it is necessary to study the structure at the cellular level.
Functions
The cortex occupies most of the cerebral hemispheres, and its thickness is not uniform over the entire surface. This feature is due to the large number of connecting channels with the central nervous system (CNS), which ensure the functional organization of the cerebral cortex.
This part of the brain begins to form during fetal development and is improved throughout life, by receiving and processing signals coming from the environment. Thus, it is responsible for performing the following brain functions:
- connects the organs and systems of the body with each other and the environment, and also ensures an adequate response to changes;
- processes incoming information from motor centers using mental and cognitive processes;
- consciousness and thinking are formed in it, and intellectual work is also realized;
- controls speech centers and processes that characterize the psycho-emotional state of a person.
In this case, data is received, processed, and stored thanks to a significant number of impulses passing through and generated in neurons connected by long processes or axons. The level of cell activity can be determined by the physiological and mental state of the body and described using amplitude and frequency indicators, since the nature of these signals is similar to electrical impulses, and their density depends on the area in which the psychological process occurs.
It is still unclear how the frontal part of the cerebral cortex affects the functioning of the body, but it is known that it is little susceptible to processes occurring in the external environment, therefore all experiments with the influence of electrical impulses on this part of the brain do not find a clear response in the structures . However, it is noted that people whose frontal part is damaged experience problems communicating with other individuals, cannot realize themselves in any work activity, and they are also indifferent to their appearance and outside opinions. Sometimes there are other violations in the performance of the functions of this body:
- lack of concentration on everyday objects;
- manifestation of creative dysfunction;
- disorders of a person’s psycho-emotional state.
The surface of the cerebral cortex is divided into 4 zones, outlined by the most distinct and significant convolutions. Each part controls the basic functions of the cerebral cortex:
- parietal zone - responsible for active sensitivity and musical perception;
- the primary visual area is located in the occipital part;
- the temporal or temporal is responsible for speech centers and the perception of sounds coming from the external environment, in addition, it is involved in the formation of emotional manifestations, such as joy, anger, pleasure and fear;
- The frontal zone controls motor and mental activity, and also controls speech motor skills.
First and second signaling systems
The role of the cerebral cortex in improving the first signaling system and developing the second is invaluable. These concepts were developed by I.P. Pavlov. The signaling system as a whole is understood as the entire set of processes of the nervous system that carry out perception, processing of information and the response of the body. It connects the body with the outside world.
The first signaling system determines the perception of sensory-specific images through the senses. It is the basis for the formation of conditioned reflexes. This system exists in both animals and humans.
In the higher nervous activity of man, a superstructure has developed in the form of a second signaling system. It is peculiar only to humans and is manifested by verbal communication, speech, and concepts. With the advent of this signaling system, abstract thinking and generalization of countless signals from the first signaling system became possible. According to I.P. Pavlov, words turned into “signals of signals.”
The emergence of the second signaling system became possible thanks to complex labor relationships between people, since this system is a means of communication and collective work. Verbal communication does not develop outside of society. The second signaling system gave rise to abstract (abstract) thinking, writing, reading, counting.
Words are perceived by animals, but completely differently from people. They perceive them as sounds, and not their semantic meaning, like humans. Therefore, animals do not have a second signaling system. Both human signaling systems are interconnected. They organize human behavior in the broad sense of the word. Moreover, the second changed the first signaling system, since the reactions of the first began to largely depend on the social environment. A person has become able to control his unconditioned reflexes, instincts, i.e. first signaling system.
Functions of the cerebral cortex
Familiarity with the most important physiological functions of the cerebral cortex indicates its extraordinary importance in life. The cortex, together with the subcortical formations closest to it, is a department of the central nervous system of animals and humans.
The functions of the cerebral cortex are the implementation of complex reflex reactions that form the basis of higher nervous activity (behavior) of a person. It is no coincidence that it received the greatest development from him. The exclusive properties of the cortex are consciousness (thinking, memory), a second signaling system (speech), and a high organization of work and life in general.
Mechanisms of neuroplasticity
During a stroke, an acute disruption of the blood supply to the brain occurs (either as a result of blockage of a vessel by a blood clot - ischemic stroke, or as a result of hemorrhage - hemorrhagic). Since, along with the blood, everything that they need for life ceases to flow to the neurons, the areas of the brain where blood circulation has stopped die off. And if these are areas responsible for motor activity - for example, the motor cortex, then the patient experiences hemiparesis, decreased muscle strength on one side of the body, or hemiplegia, complete paralysis of half the body.
The restoration of motor function is carried out mainly due to the mechanisms of neuroplasticity - the ability of the brain to change under the influence of experience: to establish new connections between neurons, destroy old and unnecessary ones, and restore those lost after damage. These processes involve not only neurons, but also neuroglial cells, as well as the vascular system [17]. The activity of synapses and their number also change [18]. To activate these mechanisms, motor rehabilitation is used in medicine. However, in patients with paralysis or a high degree of paresis, the implementation of real movements is impossible, so they resort to training with a BCI based on the imagination of movements. When imagining movements, the same areas of the brain are activated that are also involved in preparing a real action and in its execution, as a result of which such neurorehabilitation becomes real [19].
Thanks to such rehabilitation training, neurons around the damaged area undergo restructuring: the volume of gray matter in the motor zone of the brain increases, and neighboring areas take on lost functions [20]. The motor areas of the undamaged hemisphere are also involved in this process.
The effectiveness of these exercises can be increased through the use of biofeedback - visual or tactile - when the patient sees on a monitor screen how well he is completing a task (imagining the movement of a limb), or when he feels vibration from a special device when he successfully completes a task.
There are also systems that provide motor feedback: for example, when a person imagines moving his right leg, setting it in motion with a special mechanism. The Biokin system (Kosima LLC), developed under the leadership of Yu.P. Gerasimenko, works on this principle. (Institute of Physiology named after I.P. Pavlov RAS) (Fig. 4) [21]. It includes feedback, functional electrical stimulation (FES) and transcutaneous electrical stimulation of the spinal cord (TESCS), which makes it a highly effective tool in the field of neurorehabilitation of the lower extremities [22].
Figure 4. Biokine. A complex for neurorehabilitation of the lower extremities, based on the use of a BCI with feedback, FES (functional electrical stimulation) and tESCS (transcutaneous electrical stimulation of the spinal cord).
Biokin website
Such systems make it possible to close the sensorimotor loop: from the efferent (outgoing) signal of motor activity sent by the brain to the afferent (incoming) signal of sensory feedback (Fig. 5) [23].
Figure 5. Neuroplasticity induced by motor imagery-based BCI use. When the motor areas of the cortex are damaged, real movement becomes impossible, so to activate the processes of neuroplasticity, only the possibility of imagining movements remains. The use of a BCI with visual and tactile feedback enhances these processes.
adapted from [23]
This rehabilitation mechanism can be explained by the concept of Hebbian plasticity: with the simultaneous activation of two neurons connected to each other, their synaptic interaction increases, which leads to more reliable contact between them (Fig. 6). If we assume that signal transmission from the motor cortex to the limb muscles has been disrupted due to stroke or injury, then simultaneous activation of the sensory and motor cortices may enhance previously inactive connections between neurons through plasticity and thus lead to restoration of motor function of the limbs [ 24].
Figure 6. Hebbian plasticity mechanism. Strengthening the synaptic interaction between two neurons occurs due to repeated stimulation of the postsynaptic cell by the presynaptic cell.
adapted from ""
Figure 7. Formation of new neural connections in the area of spinal cord injury (SCI).
adapted from [25]
When restoring motor function after spinal cord injury, the same mechanisms of neuroplasticity are involved. With such damage, some of the nerve fibers, including motor ones, are interrupted, which causes paralysis of the limbs, while some retain their integrity. Due to this, during neurorehabilitation, it is possible to activate neuroplasticity processes: intact fibers form synaptic connections with motor neurons (motoneurons), which, in turn, transmit a signal to the muscles (Fig. 7) [25].
To increase the effectiveness of neurorehabilitation using BCIs, functional electrical muscle stimulation (FES) is often additionally used. It provides contraction of a muscle at the moment when the user imagines a movement involving this muscle (Fig. [26]. This leads to increased neuroplasticity via the Hebbian mechanism: there is a simultaneous activation of the motor areas of the brain, which transmit signals to the motor neurons of the spinal cord, and sensory neurons, activated by a muscle contracting under the influence of FES, which closes the sensorimotor loop.
It provides contraction of a muscle at the moment when the user imagines a movement involving this muscle (Fig. [26]. This leads to increased neuroplasticity via the Hebbian mechanism: there is a simultaneous activation of the motor areas of the brain, which transmit signals to the motor neurons of the spinal cord, and sensory neurons, activated by a muscle contracting under the influence of FES, which closes the sensorimotor loop.
Figure 8. IMC-FES system. When imagining movements, a signal from the motor cortex is processed by a computer (PC) and transmitted to a functional electrical stimulation (FES) device, which causes contraction of the corresponding muscle. The signal from the muscle is then transmitted to the sensory cortex, providing feedback.
adapted from [26]
Features of the structure of the cerebral cortex
The anatomical structure of the cerebral cortex determines its characteristics and allows it to perform the functions assigned to it. The cerebral cortex has the following number of distinctive features:
- neurons in its thickness are arranged in layers;
- nerve centers are located in a specific place and are responsible for the activity of a certain part of the body;
- the level of activity of the cortex depends on the influence of its subcortical structures;
- it has connections with all underlying structures of the central nervous system;
- the presence of fields of different cellular structure, which is confirmed by histological examination, while each field is responsible for performing some higher nervous activity;
- the presence of specialized associative areas makes it possible to establish a cause-and-effect relationship between external stimuli and the body’s response to them;
- the ability to replace damaged areas with nearby structures;
- This part of the brain is capable of storing traces of neuronal excitation.
The large hemispheres of the brain consist mainly of long axons, and also contain in their thickness clusters of neurons that form the largest nuclei of the base, which are part of the extrapyramidal system.
As already mentioned, the formation of the cerebral cortex occurs during intrauterine development, and at first the cortex consists of the lower layer of cells, and already at 6 months of the child all structures and fields are formed in it. The final formation of neurons occurs by the age of 7, and the growth of their bodies is completed at 18 years.
An interesting fact is that the thickness of the cortex is not uniform over its entire length and includes a different number of layers: for example, in the area of the central gyrus it reaches its maximum size and has all 6 layers, and sections of the old and ancient cortex have 2 and 3 layers. x layer structure, respectively.
The neurons of this part of the brain are programmed to restore the damaged area through synoptic contacts, so each of the cells actively tries to restore damaged connections, which ensures the plasticity of neural cortical networks. For example, when the cerebellum is removed or dysfunctional, the neurons connecting it with the terminal section begin to grow into the cerebral cortex.
analyzer, closure apparatus of conditioned reflex connections and working device. Weakness of the closure function of the cortex and trace manifestations can be observed in children with severe mental retardation, when the formed conditioned connections between neurons are fragile and unreliable, which entails learning difficulties.
The cerebral cortex includes 11 areas consisting of 53 fields, each of which is assigned its own number in neurophysiology.
Regions and zones of the cortex
The cortex is a relatively young part of the central nervous system, developing from the terminal part of the brain. The evolutionary development of this organ occurred in stages, so it is usually divided into 4 types:
- The archicortex or ancient cortex, due to the atrophy of the sense of smell, has turned into the hippocampal formation and consists of the hippocampus and its associated structures. With its help, behavior, feelings and memory are regulated.
- The paleocortex, or old cortex, makes up the bulk of the olfactory area.
- The neocortex or new cortex has a layer thickness of about 3-4 mm. It is a functional part and performs higher nervous activity: it processes sensory information, gives motor commands, and also forms conscious thinking and human speech.
- The mesocortex is an intermediate version of the first 3 types of cortex.
The cerebral cortex has a complex anatomical structure and includes sensory cells, motor neurons and internerons, which have the ability to stop the signal and be excited depending on the received data. The organization of this part of the brain is built according to the columnar principle, in which the columns are divided into micromodules that have a homogeneous structure.
The basis of the micromodule system is made up of stellate cells and their axons, while all neurons react equally to the incoming afferent impulse and also send an efferent signal synchronously in response.
The formation of conditioned reflexes that ensure the full functioning of the body occurs due to the connection of the brain with neurons located in various parts of the body, and the cortex ensures synchronization of mental activity with the motor skills of organs and the area responsible for analyzing incoming signals.
Signal transmission in the horizontal direction occurs through transverse fibers located in the thickness of the cortex, and transmit the impulse from one column to another. Based on the principle of horizontal orientation, the cerebral cortex can be divided into the following areas:
- associative;
- sensory (sensitive);
- motor.
When studying these zones, various methods of influencing the neurons included in its composition were used: chemical and physical stimulation, partial removal of areas, as well as the development of conditioned reflexes and registration of biocurrents.
The associative zone connects incoming sensory information with previously acquired knowledge. After processing, it generates a signal and transmits it to the motor zone. In this way, it is involved in remembering, thinking, and learning new skills. Association areas of the cerebral cortex are located in proximity to the corresponding sensory area.
The sensitive or sensory area occupies 20% of the cerebral cortex. It also consists of several components:
- somatosensory, located in the parietal zone, is responsible for tactile and autonomic sensitivity;
- visual;
- auditory;
- taste;
- olfactory.
Impulses from the limbs and organs of touch on the left side of the body enter along afferent pathways to the opposite lobe of the cerebral hemispheres for subsequent processing.
Neurons of the motor zone are excited by impulses received from muscle cells and are located in the central gyrus of the frontal lobe. The mechanism of data receipt is similar to the mechanism of the sensory zone, since the motor pathways form an overlap in the medulla oblongata and follow to the opposite motor zone.
The cerebral cortex is formed by several layers of neurons. A characteristic feature of this part of the brain is a large number of wrinkles or convolutions, due to which its area is many times greater than the surface area of the hemispheres.
Cortical architectonic fields determine the functional structure of areas of the cerebral cortex. All of them are different in morphological characteristics and regulate different functions. In this way, 52 different fields are identified, located in certain areas. According to Brodmann, this division looks like this:
- The central sulcus separates the frontal lobe from the parietal region; the precentral gyrus lies in front of it, and the posterior central gyrus lies behind it.
- The lateral groove separates the parietal zone from the occipital zone. If you separate its side edges, you can see a hole inside, in the center of which there is an island.
- The parieto-occipital sulcus separates the parietal lobe from the occipital lobe.
The core of the motor analyzer is located in the precentral gyrus, while the upper parts of the anterior central gyrus belong to the muscles of the lower limb, and the lower parts belong to the muscles of the oral cavity, pharynx and larynx.
The right-sided gyrus forms a connection with the motor system of the left half of the body, the left-sided one - with the right side.
The posterior central gyrus of the 1st lobe of the hemisphere contains the core of the tactile sensation analyzer and is also connected to the opposite part of the body.
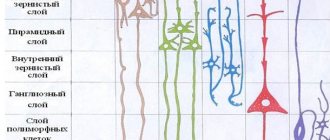
Cell layers
The cerebral cortex carries out its functions through neurons located in its thickness. Moreover, the number of layers of these cells may differ depending on the area, the dimensions of which also vary in size and topography. Experts distinguish the following layers of the cerebral cortex:
- The surface molecular layer is formed mainly from dendrites, with a small inclusion of neurons, the processes of which do not leave the boundaries of the layer.
- The external granular consists of pyramidal and stellate neurons, the processes of which connect it with the next layer.
- The pyramidal layer is formed by pyramidal neurons, the axons of which are directed downward, where they break off or form associative fibers, and their dendrites connect this layer with the previous one.
- The internal granular layer is formed by stellate and small pyramidal neurons, the dendrites of which extend into the pyramidal layer, and its long fibers extend into the upper layers or descend down into the white matter of the brain.
- The ganglion consists of large pyramidal neurocytes, their axons extend beyond the cortex and connect various structures and sections of the central nervous system with each other.
The multiform layer is formed by all types of neurons, and their dendrites are oriented into the molecular layer, and axons penetrate the previous layers or extend beyond the cortex and form associative fibers that form a connection between gray matter cells and the rest of the functional centers of the brain.
PsyAndNeuro.ru
The history of the study of psychomotor disorders since its beginning in the early years of the 19th century is a complex and confusing sequence of successive paradigms and concepts. It all started with Wilhelm Griesinger, who first introduced the concept of “psychomotor disorders.” However, his followers combined slightly different things under this term: Emil Kraepelin - sensorimotor disorders, Karl Kahlbaum and August Hoch - the psychomotor concept of catatonia, Gilles de la Tourette - involuntary movements, Jean-Martine Charcot and Pierre Marie - motor symptoms of hysterical paralysis. Carl Wernicke and Karl Kleist then described the so-called “motor” and “cycloid” psychoses as two distinct episodic states with their own psychomotor manifestations. However, in a surprisingly short time - with the advent of the first antipsychotics in the 1950s - the concept of truly psychomotor disorders was practically forgotten, and “all roads began to lead” to exclusively motor disorders. It was only in the 1960s that Karl Leonhard (a student of Karl Kleist) began to return attention to psychomotor disorders themselves.
Although psychomotor impairment is one of the key features of conditions such as major depressive disorder (MDD), bipolar affective disorder (BD) and schizophrenia, the generally accepted modern diagnostic classifications (ICD-10 and DSM-5) emphasize cognitive , affective and social symptoms, but not psychomotor ones. The RDoC dimensional classification still addresses motor symptoms of mental illness, but still places an emphasis on exclusively motor-related changes in dopaminergic cortico-striato-pallido-thalamo-cortical motor pathways. In contrast to these approaches, in our work we set a goal to revive the concept of a truly “psycho-motor” in the literal sense of the word, showing specific psychomotor (and not just motor) mechanisms.
What exactly do we mean by the term “psychomotor”? Obviously, this word consists of two parts: “psycho” and “motor”. In contrast to the reduction of the first component, i.e. “psyche”, for the sake of the second, i.e. “motor”, as often happens, we tried to directly assess the direct connection between mental and motor functions and consider the neurobiological mechanisms underlying this connection. At the neuronal level, psychomotor mechanisms determine how primary motor functions (i.e., dopaminergic subcortical-cortical pathways) are modulated by non-motor functions, i.e., cognitive and emotional systems. From a psychological point of view, psychomotor mechanisms determine body movements as a result of some mental activity, which is necessary for interaction with the environment and objects. This view is consistent with the original understanding of “psychomotor,” which emphasizes the very interaction of affective, cognitive, and motor functions. As indicated in the title of our work, we will show the pathophysiological mechanisms by which affective and cognitive changes that are not connected to motor areas in one way or another (i.e., by different roads) lead to dysfunction of the motor cortex and, accordingly, to the appearance of psychomotor symptoms .
In our work, we sought to revive the traditional understanding of psychomotor phenomena as something more than just motor disorders. Indeed, according to recent research, the neuronal and biochemical mechanisms underlying psychomotor disorders affect a much larger number of brain systems than just the dopaminergic basal-thalamo-cortical motor loop. We built our review not according to the traditional scheme, starting from nosologies, but, rather, following the RDoC concept, we grouped information according to various psychomotor syndromes.
In this paper, we present the results of MRI studies of MDD, bipolar disorder and schizophrenia, dedicated to the search for neurobiological correlates of psychomotor phenomena. We first focus on how exactly the dopaminergic subcortical-cortical motor system is modulated by other neurotransmitter systems, in particular the serotonin pathways (subcortical-cortical modulation). We then describe how the sensorimotor network (SMN) and related motor functions are modulated by other non-motor networks, in particular the brain's default mode network (DMN). We also describe how global cortical activity influences local motor cortical activity (i.e., corticocortical modulation).
Changes in the raphe nucleus modulate the activity of subcortical-cortical motor pathways
One important brainstem region influencing the dopaminergic motor network is the serotonergic raphe nucleus (RN). Since at present a fairly small number of studies have been published on the direct influence of the raphe nucleus on the motor cortex, in this section we also present data from fMRI studies of the raphe nucleus itself.
Han et al. in their study, they studied resting-state functional connectivity, rsFC (this term refers to the temporal correlation of changes in the activity of different areas of the brain; that is, two areas are considered more functionally connected if their excitation patterns are more synchronized, provided , that both of these areas are parts of the same functional network - the raphe nucleus) with other areas of the brain in patients with bipolar disorder and MDD. It turned out that in bipolar disorder and in MDD, opposite patterns of rsFC of the raphe nucleus with other subcortical regions, in particular with the thalamus and putamen, are observed. In addition, in bipolar disorder, rsFC of the hypothalamus was increased, and in depression, on the contrary, it was decreased. These findings were supported by the results of a study by Anand et al., which found a significant decrease in rsFC of the raphe nucleus with the prefrontal cortex and middle cingulate cortex in MDD. In addition, rsFC of the raphe nucleus with the hippocampus and amygdala was correlated with the severity of depressive symptoms. Finally, Wohlschlager et al. went further with rsFC studies and studied the spectral characteristics of infraslow oscillations (0.01-0.1 Hz) in the activity of the raphe nucleus and ventral tegmental area (VTA) in patients with MDD who did not receive therapy. It was found that in both areas there was a significant increase in lower frequency activity, which also directly correlated with the severity of depressive symptoms.
All these data indicate that in depression there are certain changes in the raphe nucleus itself, as well as in its connections with subcortical motor areas (thalamus and basal ganglia) and various non-motor areas of the cortex. However, at present there are very few studies devoted to possible functional changes in the raphe nucleus and its rsFC, as well as serotonergic dysfunction, in schizophrenia. However, the above studies suggested that abnormalities in the functional connections of the raphe nucleus (as the central structure of the serotonergic system) with other subcortical and cortical regions may be transdiagnostic phenomena. All this leaves open the question of the connection of these abnormalities with motor function and especially with psychomotor symptoms.
Modulation of dopamine subcortical-cortical motor pathways by the raphe nucleus and serotonin in the healthy brain.
Does the serotonin raphe nucleus influence dopaminergic subcortical-cortical pathways and motor functions? Conio et al. based on pharmacological, structural and functional MRI studies, demonstrated the presence of complex interactions between serotonin and dopamine, as well as the influence of these interactions on brain activity. In particular, it has been shown that the dopaminergic substantia nigra is connected mainly with the sensorimotor network (and the VTA with the salience network (SAN). However, the serotonergic raphe nucleus has connections with both the sensorimotor network and the passive mode network brain. In addition to the various connections between the dopamine and serotonin nuclei of the brainstem, the activity of dopamine pathways leads to increased SMN activity, and the activity of serotonergic pathways, on the contrary, leads to a decrease in SMN activity and an increase in DMN activity. Moreover, substantia nigra rsFC was directly correlated with SMN activity, and rsFC of the raphe nucleus was inversely correlated with SMN activity.Martino et al., in their study showed that in healthy subjects, rsFC between the thalamus and SMN was modulated by functional connections from both the raphe nucleus and the substantia nigra, but in the opposite way. In other words, connections from the substantia nigra contribute to greater synchronization of activity between the thalamus and SMN, and connections from the raphe nucleus, on the contrary, reduce this synchronization. Thus, based on these data, it can be argued that serotonin in the raphe nucleus and dopamine in the substantia nigra have diametrically opposite effects on the activity of the motor cortex, in particular on the SMN. Dopamine signals increase SMN activity and its synchronization with the activity of the thalamus, while serotonin signals, on the contrary, lead to “anti-correlation” of the SMN and thalamus and a decrease in SMN activity.
Thus, in answer to the question posed at the beginning of this section, it can be argued that the raphe nucleus and serotonin do indeed modulate dopamine subcortical-cortical pathways. Accordingly, dopamine pathways from the substantia nigra and serotonin pathways from the raphe nucleus may not be regarded as primary motor pathways per se, but rather as modulators of psychomotor functions.
Modulation of dopamine subcortical-cortical motor pathways by the raphe nucleus and serotonin in depression, bipolar disorder and schizophrenia.
Based on data obtained in healthy subjects, it can be assumed that psychomotor inhibition, which is based on reduced SMN activity, should be due to a decrease in substantia nigra rsFC and/or an increase in raphe nucleus rsFC. Similar phenomena are indeed observed in patients with psychomotor inhibition in the depressive phase of bipolar disorder. Such patients are characterized by a decrease in rsFC of the thalamus and SMN and substantia nigra with the thalamus/basal ganglia, as well as a competitive decrease in rsFC of the raphe nucleus with the basal ganglia/thalamus. Together, these patterns promote “decoupling,” that is, desynchronization of thalamic and SMN activity, with subsequent inhibition of SMN activity and the occurrence of psychomotor inhibition. These findings are consistent with the results of a study by Yin et al., which demonstrated decreased blood flow in the motor cortex during psychomotor inhibition in patients with MDD.
For patients with mania, on the contrary, almost diametrically opposite changes are characteristic. These patients showed a decrease in rsFC of the raphe nucleus with the thalamus/basal ganglia, and also, unlike patients with depression, rsFC of the substantia nigra was preserved. Taken together, this leads to an increase in rsFC of the thalamus with the SMN, increased SMN activity and subsequent psychomotor agitation.
Thus, the data from these studies suggest a reciprocal balance between the dopaminergic connections of the basal ganglia/thalamus to the SMN on the one hand and the serotonergic modulation of these connections by the raphe nucleus on the other hand. A decrease in the activity of serotonergic pathways from the raphe nucleus to the basal ganglia leads to greater “connectivity” of the thalamus with the SMN, which leads to psychomotor agitation. A decrease in the activity of dopaminergic pathways from the substantia nigra to the basal ganglia, on the contrary, reduces the “connectivity” of the thalamus with the SMN and leads to the occurrence of psychomotor inhibition. If these two arms of modulation of thalamus-SMN connections, dopaminergic and serotonergic, are balanced, then psychomotor function is “normal”, that is, neither accelerated nor slowed down. If this balance is disturbed, then the thalamus and SMN become either overly or underfunctionally connected, leading to either psychomotor agitation (for example, in mania) or psychomotor inhibition (in depression). See Fig. for details. 1A.
Rice. 1. Biochemical and neuronal modulation of subcortical-cortical and cortico-cortical mechanisms of psychomotor phenomena. A. Modulation of the dopaminergic subcortical-cortical motor circuit by the raphe nucleus and serotonin. B. Modulation of the sensorimotor network (SMN) by the passive mode network (DMN). C. Modulation of the display of general activity in the SMN. SN – substantia nigra, RN – raphe nucleus, Thal – thalamus.
Modulation of the dopamine subcortical-cortical motor loop by the raphe nucleus/serotonin and other neurotransmitter systems - a dimensional and transnosological approach
The next question to be answered is: can serotonin modulation of the motor loop be correlated with different diagnostic categories, or, put another way, can it be correlated with different degrees of psychomotor impairment? A kind of “litmus test” that can help answer these questions is the phenomenon of psychomotor agitation in depression. Martino et al. We separately studied patients in the depressive phase of bipolar disorder with psychomotor agitation and compared them with corresponding patients with psychomotor inhibition. It turned out that in depressed patients with psychomotor agitation, a pattern similar to that observed in mania is observed: in both conditions, there is an increase in rsFC of the thalamus with the SMN, which, accordingly, leads to increased SMN activity and psychomotor agitation.
Further evidence of the dimensional and transnosological nature of bilateral “from the raphe nucleus and from the substantia nigra) modulation of the activity of the subcortical-cortical motor loop follows from the results of a recent study of schizophrenia. The authors studied two groups of patients with schizophrenia - with psychomotor agitation and with psychomotor inhibition - and compared them, respectively, with groups of patients with bipolar disorder in the manic phase (and with psychomotor agitation) and in the depression phase (with concomitant psychomotor inhibition). It turned out that patients suffering from schizophrenia with psychomotor inhibition are characterized by almost the same pattern of changes in thalamo-sensorimotor rsFC as patients suffering from depression with psychomotor inhibition, that is, a decrease in rsFC of the thalamus with SMN and a concomitant decrease in rsFC of the raphe nucleus and nigra substances with the basal ganglia and thalamus. A similar overlap was observed in patients suffering from schizophrenia with psychomotor agitation. The increase in rsFC of the thalamus with SMN and the decrease in rsFC of only the raphe nucleus with the basal ganglia and thalamus observed in such patients are similar to the corresponding changes in patients in the manic phase of bipolar disorder with psychomotor agitation.
A textbook example of a psychomotor syndrome is catatonia, which is a truly transdiagnostic phenomenon. Catatonia can occur in schizophrenia, bipolar disorder, MDD, and other diseases. The development of catatonia is the result of dysfunction of several neurotransmitter systems. Indeed, catatonia is primarily manifested by motor disorders associated with changes in the activity of sensorimotor subcortical-cortical zones. However, catatonia is also characterized by affective disturbances associated with changes in non-motor areas of the cortex (for example, with dysfunction of frontoparietal connections), which emphasizes the truly psychomotor nature of this phenomenon. Accordingly, along with hypoactivation of dopaminergic receptors, disturbances in other neurotransmitter systems are observed in catatonia: hypoactivation of serotonin (5-HT2A) receptors, an imbalance between the activity of GABAA (hypoactivation) and GABAB (hyperactivation) receptors and, probably, also hyperactivation of glutamate NMDA receptors. On the one hand, lorazepam and zolpidem (allosteric modulators of GABAA receptors) increase the excitability of GABAergic inhibitory networks in the motor cortex and thus help alleviate the symptoms of catatonia. On the other hand, baclofen and valproic acid can increase the activity of GABA and NMDA receptors and, conversely, increase the severity of catatonia. However, there is still some evidence of a beneficial effect of valproic acid, topiramate, and carbamazepine on affective catatonic symptoms (presumably by increasing GABA and NMDA responsiveness). Finally, clozapine (a 5-HT2A receptor antagonist and GABAA receptor agonist) compensates for the serotonergic hypoactivation imbalance of GABAA-B activity and thus may reduce the severity of catatonia in some studies.
Another example of a transnosological psychomotor syndrome is parkinsonism (a combination of tremor, rigidity and bradykinesia), which is mainly a consequence of Parkinson's disease, which is primarily neurodegenerative in nature. However, the phenomena of parkinsonism can also be associated with other diseases (for example, parkinsonism can be observed in patients with schizophrenia). Parkinson's disease is primarily characterized by degeneration of dopaminergic cells in the substantia nigra and striatum, but the disease has also been found to impair the activity of the serotonergic system. Thus, in Parkinson's disease, there is a decrease in the number of serotonin transporters in the raphe nucleus, which is directly associated with the severity of tremor. In addition, other neurotransmitter systems are also affected in Parkinson's disease. It is known that GABAergic system modulators (zolpidem and clonazepam), amantadine, anticholinergic drugs and clozapine can improve the motor symptoms of Parkinson's disease. Patients with schizophrenia and Parkinsonism exhibit characteristic changes in gray matter volume and activity in frontothalamic/cerebellar and cortical somatosensory networks that are not found in patients without Parkinsonism. These facts make it possible to assume the existence of ascending modulation of the motor cortex as a central neuronal mechanism for the occurrence of parkinsonism in schizophrenia.
Finally, psychomotor impairments can also be observed in other diseases, in particular in autism. It is worth noting that there is evidence of changes in the activity of the dopaminergic, serotonergic (increased synthesis of serotonin), GABAergic and glutamatergic systems in the motor and somatosensory cortex, as well as in the striatum, associated with this disorder. Accordingly, dopamine receptor blockers, serotonin reuptake inhibitors, memantine, valproic acid, arbaclofen, and acamprosate have been shown to be effective in reducing psychomotor symptoms of autism such as stereotypies, impulsivity, and irritability in some studies.
Taken together, these results clearly demonstrate that modulation by the raphe nucleus and serotonin of the subcortical-cortical motor loop represents a phenomenon that extends beyond any specific disease category. It corresponds rather to the direction and severity of psychomotor disorders, rather than to any specific diagnosis, thus being a truly dimensional and transnosological phenomenon.
Modulation of the motor loop by other cortical networks
The cerebral cortex includes various neural networks: the passive mode network (DMN), sensory networks (visual, auditory, somatosensory), sensorimotor network (SMN), frontoparietal network, salience network, attention networks and many others. Recent studies have shown that all these networks are functionally interconnected. For example, the DMN and the frontoparietal network are opposite in their activity: a functional increase in the activity of one directly leads to inhibition of the activity of the other and vice versa. Most interestingly, the SMN also appears to be in reciprocal relationships with other networks, particularly the DMN and sensory networks. In a study by Martino et al. It has been demonstrated that an increase in neuronal variability in the DMN is associated with a decrease in this parameter in the SMN, even in healthy individuals.
Just as in the case of subcortical modulation, this reciprocal interaction is extremely pronounced in patients with bipolar disorder in the phase of depression and mania. Patients in the depressive phase exhibit abnormally high neuronal variability in the DMN, which, following a reciprocal mechanism, was accompanied by a decrease in neuronal variability in the SMN. Changes in these parameters correlated with the severity of symptoms: the greater the neuronal variability in the DMN compared to the SMN, the more severe the depressive symptoms were. Interestingly, in patients in the manic phase, on the contrary, neuronal variability in the DMN was reduced and, accordingly, increased in the SMN. This also correlated with manic symptoms: the greater the variability of the SMN compared to the DMN, the more severe the patient's manic symptoms. It is worth emphasizing that it was the ratio of DMN/SMN variability that correlated with the severity of symptoms, but not the absolute values of these indicators themselves.
In another study, Northoff et al. examined neuronal variability in the SMN and visual network in patients with bipolar disorder. The authors found that during mania, an increase in variability in the SMN was accompanied by a decrease in that in the visual network. The opposite picture was observed in depression: a decrease in variability in the SMN and an increase in that in the visual network. The authors associated this balance with “internal” and “external” perceptions of time speed. “Internal” perception was determined by the activity of the SMN and the subcortical-cortical motor loop, while “external” perception was determined by sensory areas, including the visual network. If we take the degree of neuronal variability as an indicator reflecting the perception of time (increased variability indicates a greater change in neuronal activity per unit of time and, accordingly, the “acceleration” of time at the neuronal level), then we can say that patients with depression tend to slow down “ internal" time, that is, abnormal "intrinsic slowing" (resulting from decreased neuronal variability in the SMN). In contrast, patients with mania are characterized by a subjective “acceleration” due to increased variability in the SMN. The question of how these anomalies of “internal time” are transformed into psychomotor disorders and whether they are transformed at all remains open.
These studies clearly demonstrate that neuronal activity in the motor cortex and SMN depends not only on the subcortical influences of the raphe nucleus and substantia nigra, but also on the activity of other, non-motor cortical networks. The clearest evidence for this is the reciprocal interaction between the DMN and SMN, as well as the modulation of the SMN by sensory networks. However, the mechanisms by which such reciprocal corticocortical modulation is associated with psychomotor performance remain to be explored.
Modulation of local-regional activity of the motor cortex by general cortical activity
Traditionally, we measure neuronal activity locally, that is, in a specific area (signal amplitude from the area), or in a specific network (synchronization between different parts of the network, assessed using rsFC). However, in addition to these local methods, there are also methods that assess brain activity in general. One such method is to determine rsFC between entire neural networks, for example, between the DMN and SMN. In addition, if you take all the inter-regional and inter-network connections together, you can get the so-called “global signal”, assessed using fMRI. The global signal is calculated as the average of all rsFCs across the entire brain and reflects the degree to which different regions and networks are synchronized with each other, that is, global brain synchronization. Research demonstrates that the degree to which a particular region or network is synchronized with the rest of the brain varies among different regions. For example, neuronal activity in the SMN is more synchronized with overall brain activity (and thus exhibits greater global signal on fMRI) than the DMN, whose regions appear to operate more separately, that is, desynchronized (as reflected by lower global signal on fMRI).
Currently, there are reports of global signal changes in various mental disorders. Yang et al. demonstrated that patients with schizophrenia have a significantly higher global signal compared to patients with bipolar disorder and healthy subjects. This means that global synchronization of activity between distinct brain regions/networks is abnormally high in patients with schizophrenia (however, in another study, Argyelan et al., in contrast, demonstrated decreased global synchronization in patients with schizophrenia). Moreover, it has been found that in schizophrenia, global activity becomes less synchronized with the activity of lower order regions/networks, especially sensory areas. On the contrary, global activity becomes more synchronized with the activity of higher order zones. Wang et al. supplemented these data by showing that the synchronization of individual networks with general brain activity is a dynamic, time-varying process: first, global activity is synchronized with sensory networks, then with the DMN, and then with other networks. This sequence of synchronization of global brain activity with distinct regions/networks appears to be disrupted in schizophrenia.
In another study, Zhang et al. studied the global signal in bipolar patients in manic, depressive and euthymic phases. It was found that in patients with bipolar disorder in the depressive phase there is increased synchronization of global brain activity with the hippocampus, which may be associated with more frequent “memory recall” of autobiographical memories. In addition, it turned out that in manic patients, global activity is highly synchronized with the activity of the motor cortex, which reflects the greater psychomotor activity characteristic of these patients. The latter finding particularly emphasizes that synchronization of global activity is a true psychomotor mechanism: changes in the motor cortex cause changes in overall brain activity unrelated to the subcortical-cortical motor loop.
This review has the following important limitations. Firstly, the reviewed works differ greatly from each other in terms of diagnostic groups, approaches to the definition of psychomotor disorders and neuroimaging techniques. Secondly, the variety of approaches and methods, coupled with a small number of studies, did not give us the opportunity to conduct a full-fledged ALE-meta-analysis (activation likelihood estimation - an approach to meta-analysis of neuroimaging studies, which consists in determining the likelihood that areas of high the activities of the cortex really coincide - approx.). Third, due to the diversity of terminology related to the concept of “psychomotor,” we may have missed some important studies. Based on the reasons listed above, we consider it necessary to conduct further transdiagnostic longitudinal neuroimaging studies of psychomotor disorders using standardized methods.
This review has demonstrated the different neuronal mechanisms underlying psychomotor symptoms in various mental illnesses. This is a kind of continuation of the classical view of psychomotor syndromes, which is relevant at the present time, since it has been demonstrated that the neuronal and biochemical mechanisms of the occurrence of psychomotor disorders are not limited to the dopamine subcortical-cortical motor loop. We identified three transdiagnostic neurobiological mechanisms underlying psychomotor performance. 1) modulation by serotonin and the raphe nucleus of the dopaminergic subcortical-cortical motor loop; 2) reciprocal balance between the passive mode network of the brain, sensorimotor and sensory networks; 3) local synchronization of the sensorimotor network with global brain activity.
These mechanisms are fundamentally quite similar. Firstly, all three mechanisms depend not on any absolute values, but on relative indicators, that is, on the balance between any parameters. We encountered three types of neural balance, relating to different levels of manifestation of psychomotor functions: 1) balance between rsFC of the raphe nucleus and the substantia nigra; 2) balance between the activity of the passive mode network of the brain and the somatosensory network; 3) balance between global brain activity and local activity of the somatosensory network. In addition, we have identified different types of biochemical balance, for example between dopamine and serotonin, shaping psychomotor functions through modulation of subcortical-cortical and cortico-cortical neural balances.
Secondly, the data we analyzed indicate the dimensional and transnosological nature of psychomotor mechanisms that occur not only in various diseases (for example, schizophrenia, bipolar disorder or depression), but also in normal conditions. Accordingly, psychomotor mechanisms illustrate the advantages of using a dimensional transnosological syndromic approach (as done, in particular, by the authors of RDoc)
Third, these examples demonstrate a continuum of healthy and pathological psychomotor states in which the same mechanism is involved, but manifested to varying degrees. Within this continuum, the normal corresponds to the average values, while the pathological conditions are located at opposite ends, thus forming an inverted U-shaped curve (see Fig. 2)
Fig.2. Inverted U-shaped continuum curves of different neurobiological mechanisms of psychomotor activity. A. A continuum of different types of balance between the functional connectivity of the raphe nucleus (RN) and substantia nigra (SN) with the thalamus (Thal) and its connection with the sensorimotor network (SMN), which determines its activity. B. A continuum of different types of balance between SMN activity and the passive mode network (DMN) that determines the level of SMN activity. C. Continuum of different types of balance between global brain activity (GA) and level of SMN activity.
Fourth, the inverted U-shaped curve demonstrates that it is the average indicators of various types of neurobiological balance that are most optimal. In contrast, extreme forms of imbalance are dysfunctional, leading to impaired psychomotor functions.
Fifth, from a clinical point of view, psychomotor disorders can be characterized by a specific set of symptoms, that is, a combination of certain motor, affective and cognitive disorders. For example, psychomotor agitation may be accompanied by emotional disturbances (eg, increased affect) and cognitive impairments (particularly impaired attention). At the same time, psychomotor inhibition is often accompanied by negative emotions and increased attention to oneself. Such symptom constructs suggest the existence of connections between the subcortical-cortical motor system and non-motor, affective and cognitive neural systems, as evidenced, for example, by the existence of reciprocal interactions between the SMN, DMN and sensory networks.
Finally, all of these mechanisms have potential diagnostic and therapeutic implications. They can be used for early diagnosis of borderline states and manifestations of mental illness, serve as potential biomarkers of therapeutic response and be targets of non-invasive methods of brain stimulation (transcranial magnetic stimulation, etc.) Despite the fact that these methods can stimulate different parts of the brain, all of them will in one way or another affect the activity of the subcortical-cortical motor loop and reduce the severity of psychomotor disorders. As with Rome, all roads ultimately lead to the motor cortex.
Author of the translation: Kibitov A.A.
Source: Northoff, G., Hirjak, D., Wolf, RC et al. All roads lead to the motor cortex: psychomotor mechanisms and their biochemical modulation in psychiatric disorders. Mol Psychiatry (2020). https://doi.org/10.1038/s41380-020-0814-5