The brain is responsible for any processes in the body, including muscle function. It is from this that the signal is transmitted about certain muscle contractions, which ultimately leads to movement.
In some cases, the nerve impulse may be blocked, resulting in seizures. Most often they are diagnosed in childhood, usually due to immaturity of brain cells, but sometimes problems with muscle functionality can also be the cause. To combat the consequences of this phenomenon, and most importantly, to prevent it, the Doctor Choi clinic uses a unique combination of modern European techniques and traditional oriental medicine. Special acupressure, acupuncture and homeopathic remedies can protect the child from this extremely unpleasant and dangerous problem. In addition, with such exposure, a general strengthening of the body occurs, which makes it possible to prevent many childhood diseases.
Types of convulsive reactions
In order to prescribe treatment, it is necessary to determine the main type of convulsive process:
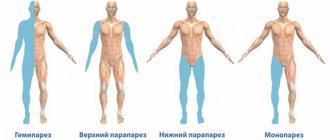
- localized - cause muscle contraction in a specific part of the body. Most often, children experience leg cramps. Causes and treatment are determined by the results of examination and tests;
- generalized – muscle contraction is observed throughout the child’s body. Most often, the cause of this is a disorder in the hemispheres of the brain responsible for movement function.
- In addition, there are subtypes of seizures:
- clonic - usually observed during sleep;
- tonic is a contraction of muscle tissue that is diagnosed over a long period of time. If they are present, there is a lack of speech;
- atonic – there is no muscle tone.
All these types of convulsive processes are divided into two broad groups:
- epileptic seizures in children - as the name implies, epilepsy is considered the root cause of their occurrence. Symptoms such as attacks of nausea, chills, fever may occur;
- non-epileptic - the cause may be heart disease, leukemia or hemophilia.
When a convulsive syndrome appears in children, emergency assistance must arrive on time. Parents should closely monitor their child and immediately contact a doctor at the first symptoms of seizures.
Generalized convulsive status epilepticus
Status epilepticus (SE) is a condition lasting 5 minutes or more and corresponding to one of the following definitions:
- ongoing clinical or electroencephalographic seizure activity;
- repeated seizure activity without recovery (return to baseline) between seizures.
There are the following types of classification of ES.
According to the degree of involvement of brain substances in seizure activity:
- generalized (attack activity in both hemispheres, usually absence or severe depression of consciousness);
- partial (seizure activity in one hemisphere, usually intact or partial impairment of consciousness).
By external manifestations:
- convulsive;
- non-convulsive.
According to pathophysiological changes:
- ES in the compensation phase (first 30 min);
- ES in the decompensation phase (after 30 min).
Based on response to therapy:
- curable (cessation of status after initial administration of a benzodiazepine and/or intravenous antiepileptic drug (AED));
- refractory (lack of clinical effect after administration of a benzodiazepine and/or intravenous AED).
By type of attack:
- simple partial seizure status;
- status of complex partial seizures;
- absence seizure status;
- generalized seizure status;
- status of myoclonic seizures;
- other rare status types.
By etiology:
- ES due to an actively ongoing acute process;
- ES as a result of a chronic disease or damage to the nervous system (due to remote causes);
- febrile ES in children;
- epilepsy de novo, debuting with status.
Generalized convulsive epileptic
status (GSES) is the most common and most dangerous type of status epilepticus. Seizure activity during HSES causes severe metabolic disorders that pose a threat to the patient's life. The following stages of GSES are distinguished:
Overt GCSE - phase I of GSES, characterized by pronounced motor manifestations (usually the first 30 minutes of ES), hereinafter referred to as “full-scale GSES”.
Subtle GCSE - II phase of GSES, the main signs of which are: extinction of motor activity and progressive disruption of homeostasis parameters (usually this phase occurs 30-60 minutes after the start of ES), hereinafter referred to as “elusive GSES”.
Electrical GCSE - III phase of GSES, in which there is a lack of motor activity, gross decompensation of vital functions and pathological electrical activity that persists on the EEG (usually after the first 120 minutes of status), hereinafter referred to as “electrical GCSE”.
Epidemiology
The incidence of ES ranges from 10.3 to 61 per 100 thousand people, it is maximum in children of the first year of life (135–165 per 100 thousand population) and elderly patients (14.6–86 per 100 thousand population). The frequency of GSES (both primary and secondary generalized attacks) ranges from 33.3% to 71% of all cases of SE. Thus, at least 200 thousand cases of HSES occur annually in the world.
Etiology
HSES can be caused by various reasons. In 12% of cases, epilepsy debuts with status.
Acute processes that may underlie ES:
- metabolic disorders: electrolyte abnormalities, hypoglycemia, renal failure;
- sepsis;
- CNS infections: meningitis, encephalitis, abscess;
- stroke: ischemic stroke, intracerebral or subarachnoid hemorrhage, sinus thrombosis;
- traumatic brain injury with or without epidural or subdural hematoma;
- Drug-related SE: drug toxicity as a cause of SE;
- stopping taking opiates, benzodiazepines, barbiturates, ethanol;
- non-compliance (discontinuation/reduction of AED dose);
- hypoxia, cardiac arrest;
- hypertensive encephalopathy, posterior reversible encephalopathy syndrome;
- autoimmune encephalitis (anti-NMDA antibodies, antibodies to voltage-dependent potassium channels, paraneoplastic syndromes).
Chronic processes that may underlie ES:
- patients with epilepsy: return of seizures or discontinuation of AEDs;
- long-term use of ethanol: alcohol intoxication or cessation of alcohol intake;
- central nervous system tumors;
- long-term consequences of central nervous system lesions (stroke, abscess, trauma, cortical dysplasia).
Features of ES in children:
- In young children with ES, acute symptomatic ES is common;
- prolonged febrile seizures are the most common cause of ES in children (more than 40% of all cases of ES);
- CNS infections, especially bacterial meningitis, inborn errors of metabolism, and malabsorption are common causes of ES in children.
Clinical manifestations
HSES is characterized by paroxysmal or permanent motor activity. It can be tonic, clonic or tonic-clonic, symmetrical or asymmetrical, clearly visible or weak. However, it is always associated with marked impairment of consciousness and bilateral (although often asymmetric) ictal discharges on the EEG.
The stage of SE, which is called "full-scale GSES", usually begins with a series of discrete generalized seizures characterized by tonic, clonic or tonic-clonic motor activity, which is associated with epileptiform discharges on the EEG, changing during the seizures. At this stage of status, clonic seizures suddenly stop, which coincides with the end of the attack on the EEG. As a rule, in the interictal period the patient partially regains consciousness, however, if he is not in a state of active wakefulness without any residual confusion and / or other neurological symptoms, he should be considered to have HSES continues. If at this stage the patient does not receive treatment, or it is inadequate, motor manifestations, despite the persistence of ES, become increasingly erased.
The next stage of status is called “elusive GSES”. The patient exhibits only isolated contractions of the muscles of the fingers, anterior abdominal wall, face, or nystagmoid movements of the eyeballs. If the status continues, the patient’s motor activity is not detected at all, despite the persistence of epileptiform discharges on the EEG. This - the terminal stage of the GSES - is called "electric GSES". Thus, the evolution of untreated (or inadequately treated) GSES goes from deployed to elusive GSES and ends with electric GSES. However, in some patients with severe (eg, anoxic) encephalopathy, the subtle stages of GSES are preceded by only 1-2 (and sometimes not a single) generalized epileptic seizure.
Interictal symptoms depend on the stage of GSES. Discrete attacks usually evolve from an initial phase of tonic muscle tension to their clonic contractions, increasing in amplitude and decreasing in frequency, which then pass quickly and completely. After this, the patient is in a comatose state and motionless. If this is truly an interictal period, the EEG shows low-amplitude activity that does not contain epileptiform discharges. In the case of a discrete generalized attack, the patient gradually emerges from the coma, and in parallel, EEG activity normalizes. However, even if the patient comes to his senses, and epileptiform activity continues to persist on the EEG, it is unacceptable to state the cessation of GSES. In classical cases, motor activity in HSES evolves from clinically pronounced to erased, while electroencephalographic and clinical disorders become permanent.
Diagnostics
Full-blown HSES is defined as two or more generalized seizures without full recovery of consciousness between them or as a single prolonged seizure. In the case of classic attacks, the diagnosis does not cause difficulties, but a typical mistake is underdiagnosis of the status due to the false impression that the patient is regaining consciousness in the interictal period. Any state of consciousness, with the exception of active wakefulness, is a sign that seizure-induced pathophysiological changes persist and the patient is in SE.
It should be taken into account that clinical and EEG patterns of GSES may be asymmetric. Doctors often mistakenly diagnose partial ES in patients with impaired consciousness and unilateral seizures. In such cases, GSES with asymmetrical or unilateral motor manifestations should be assumed if the patient is not fully conscious during the convulsions.
Despite the fact that the diagnosis of GSES is initially clinical, EEG data play an important role in its objectification and the choice of treatment tactics. Based on the study of experimental models of ES and studies in humans, 5 sequential EEG patterns of GSES have been described. At the initial stage, ictal discharges on the EEG correspond to generalized seizures. If the status progresses without adequate treatment, discrete discharges merge to form longer patterns with lower amplitude, which eventually become monomorphic and continuous. Such activity can be represented by spikes, spike-wave complexes, rhythmic sharp or slow waves and is subsequently interspersed with periods of flattening, which corresponds to the extinction of motor activity. In the final phase of the status, discharges of epileptiform activity are recorded on a flat background. Identification of any of these patterns in a patient in a coma confirms the diagnosis of HSES, even with erased motor manifestations.
According to generally accepted opinion, for adequate management of a patient with ES it is necessary to monitor the EEG, but the start of treatment should in no case be delayed until its results are obtained. Of course, if the attack ends and the patient fully regains consciousness, there is no need to conduct an EEG. However, if the patient, despite the cessation of seizures, has a change in the level of consciousness, it is necessary to exclude the persistence of epileptiform discharges using an EEG. An EEG is also necessary in patients who have had a single seizure but have not returned to a state of active wakefulness. It should be remembered that subtle HSES can develop after a single generalized attack.
After the episode of GSES has stopped, further diagnostic research should be focused on finding the cause of the development of the status.
Differential diagnosis
Most often, GSES requires differential diagnosis with psychogenic non-epileptic seizures. Convulsive activity in a number of patients with psychogenic status is pronounced. For the differential diagnosis of psychogenic and true ES, one should remember 4 main characteristics of the latter: the evolution of motor activity during an attack, the stereotypical nature of attacks, the absence of pauses in convulsions during an attack, open eyes during an attack. As a rule, a generalized convulsive attack consists of stereotypical phases: first - tonic, then - clonic. Severe clonic convulsions followed by tonic tension and then cessation of the attack are never observed during a true epileptic seizure, but mild clonic movements may precede the tonic phase during clonic-tonic-clonic seizures. Clonic twitching in the final phase of tonic-clonic or clonic-tonic-clonic seizures decreases in frequency and increases in amplitude and then usually stops suddenly. In the case of the spread of convulsive activity during partial seizures of the Jacksonian march type, the clinical picture should correspond to the topical organization of the motor cortex. If the development of such attacks is atypical, the question arises about their psychogenic nature. However, some attacks, especially those of frontal origin, may be characterized by unusual behavior, including strange shouting, flapping of arms, rocking of the head, or sexually suggestive movements of the pelvis. If the seizures are stereotypical in their motor manifestations, it is likely that they are truly epileptic, even despite the strange features. In contrast, psychogenic seizures often vary from one episode to the next. In addition, motor activity during psychogenic seizures is usually interspersed with short periods of rest, which is not typical for true epileptic paroxysms.
EEP plays an important role in the differential diagnosis of epileptic and psychogenic seizures. A well-modulated alpha rhythm in the posterior leads in the early post-attack period serves as convincing evidence of the psychogenic nature of the paroxysm. In turn, after generalized epileptic seizures, a slowdown in the EEP is characteristic, although after frontal seizures a rapid restoration of consciousness and normalization of the EEP are often observed
Forecast
Mortality and the formation of persistent neurological deficits due to PSES are largely determined by its etiology, but their frequency may increase with inadequate treatment. Long-term progression of the status is associated with a poor prognosis.
Nonspecific therapy for GSES
Pathophysiology of GSES
Physiological changes during PSES can be simplified into two phases; the transition from the first to the second occurs approximately 30–60 minutes after the onset of the attack.
During the compensation phase, a massive release of catecholamines occurs, which leads to an increase in heart rate, blood pressure and plasma glucose. Arrhythmias, including fatal ones, may occur. With prolonged seizures, persistent hyperthermia develops up to 40°C, leading to brain damage and worsening the prognosis. Acidosis also often develops, with arterial pH falling below 7.0 in 25% of patients. This acidosis is mainly associated with the formation of lactate, as well as with an increase in CO2 levels in the blood, which in itself can lead to life-threatening depression of consciousness. Acidosis increases the risk of arrhythmias and hypotension and, in combination with cardiovascular complications, can lead to pulmonary edema. The activity of the autonomic nervous system causes sweating, increased bronchial secretion, salivation, and vomiting.
However, physiological self-regulatory mechanisms in the brain are sufficient to compensate for these changes. There is a significant increase in cerebral blood flow and glucose delivery to active brain tissue. Interneuronal connections at this stage are not impaired, the blood-brain barrier is preserved, and the risk of brain damage is low.
During the decompensation phase, compensatory mechanisms are disrupted, which leads to a progressive decrease in blood pressure and hypoxia. Hypotension caused by autonomic and cardiovascular disorders, as well as the effects of drugs, can be very dangerous and resistant to therapy. In combination with impaired autoregulation, hypotension leads to a lack of blood supply to the brain. And during the generation of electrical discharges, the brain needs especially many nutrients. With hypoperfusion, these needs cannot be met, leading to ischemic excitotoxicity and metabolic damage. Hypotension may be exacerbated by intravenous administration of AEDs, especially if this administration is carried out too quickly. Increased intracranial pressure (ICP) in late status, aggravated by systemic hypotension, can lead to cerebral edema. However, the disorders are not limited to brain metabolism. Due to pulmonary hypertension and pulmonary edema, the pressure in the pulmonary artery exceeds the osmotic pressure of the blood, which leads to damage to the pulmonary capillaries. Cardiac output decreases due to decreased contractility of the left ventricle and a drop in stroke volume. This leads to the development of heart failure.
In general, the status leads to hyperthermia, acidosis, hypoglycemia, hyponatremia, hypo- and hyperkalemia. Acute tubular necrosis, renal and liver failure, and DIC syndrome may develop. Rhabdomyolysis during severe seizures may worsen renal failure.
Brain damage due to status. The primary goal of immediate treatment for PSES is to prevent brain damage. Status-induced brain damage has a number of mechanisms. Pypoxia, ischemia or metabolic disorders are certainly some of the causes of such damage, but excitotoxicity plays a major role. It is caused by both the electrical seizure activity itself and the repeated depolarization of neurons that occurs during continuous seizures. Electrical activity leads to a gradually increasing influx of calcium into the affected neurons, which triggers the processes of apoptosis. The result is the death of neurons, particularly in the hippocampus, but also in other areas of the brain. The process of cell death due to excitotoxicity is aggravated by energy disruptions characteristic of the decompensation phase. The longer the status lasts, the greater the risk of damage. Thus, during status it is fundamentally important to stop both motor and electrical seizure activity. If AEDs do not control both the clinical and electrical manifestations of seizures within 2 hours, general anesthesia is indicated to completely suppress neural activity.
Diagnostic and therapeutic procedures
Cardiorespiratory function is the top priority in all patients with status. It is necessary to open the airways and, if necessary, provide artificial respiration. Status pipoxia is always more severe than expected, which is associated with constant muscle activity and increased metabolic needs of the brain, therefore, in all cases, enrichment of the respiratory mixture with oxygen is indicated.
Respiratory failure can be a consequence of cardiovascular failure, accelerated metabolism, pulmonary edema, as well as respiratory depression due to drugs prescribed to treat the status. It is necessary to be constantly prepared to start mechanical ventilation, especially considering that hypoxia status is always higher than expected. The use of subtherapeutic doses of anesthetics is not recommended without connection to mechanical ventilation. Clinical practice shows that even 10 mg of intravenous diazepam can lead to respiratory depression. Aspiration pneumonia is a common complication of the condition, so broad-spectrum antibiotics are indicated for all patients requiring respiratory support.
Monitoring of pulse, blood pressure, EEG, temperature and regular assessment of neurological status are indicated in all patients. Metabolic disorders, especially hypoglycemia, can be the cause of the status or develop in parallel with it, so regular assessment of clinical and biochemical blood tests, blood gases, coagulograms, and pH is required.
Intravenous access should be provided to replace fluid loss and administer AEDs. The drugs should not be mixed, and if two AEDs (eg, diazepam and valproate) are required, two intravenous lines should be provided. Intra-arterial administration of drugs during status is strictly prohibited due to the risk of arterial wall necrosis and vasospasm.
Urgent tests. The patient should immediately take a blood test to determine gases, sugar, liver and kidney function, calcium and magnesium levels, full hemogram (including platelets), coagulogram and plasma AED levels. 20 ml of serum should be retained for further analysis, especially if the cause of the status remains unknown. Additional tests should be performed depending on the clinical situation.
Intravenous glucose and thiamine. All patients with hypoglycemia (checking glucose levels with a hand-held glucometer are recommended) are advised to immediately administer 50 ml of a 40% glucose solution. Routine administration of glucose to patients without hypoglycemia may exacerbate neuronal damage.
In patients with alcoholism or malnutrition, 250 mg of thiamine intravenously is recommended. Administration of thiamine is especially important if glucose administration is planned because in individuals with alcoholism and/or malnutrition, glucose administration increases the risk of developing Wernicke encephalopathy. Thiamine should be administered very slowly due to the risk of anaphylactic shock.
Acidosis and other metabolic disorders. Lactic acidosis is a typical complication of the status associated with convulsive activity and hyperthermia. It can usually be controlled by stopping seizure activity with antipsychotic drugs, anesthetics, or muscle relaxants. In cases of severe acidosis, bicarbonate may be administered to prevent shock, hypotension, and depression of cerebral blood flow. However, in most cases, bicarbonate administration is not required. Correction of hypoglycemia, electrolyte and other metabolic disturbances should be rapid and effective.
Magnesium sulfate is used in the treatment of eclampsia, but there is no evidence of its effectiveness in status epilepticus. However, decreased serum magnesium may occur in alcoholics or patients with AIDS. Such patients are advised to administer 2–4 g of magnesium sulfate intravenously over 20 minutes to treat attacks and prevent arrhythmias. Magnesium is also used in patients with refractory status epilepticus.
Vasopressors. Hypotension can be a consequence of both the status itself and drug therapy; it is a universal problem with long-term status. Hypotension increases the risk of brain injury because loss of autoregulation of cerebral blood flow means that cerebral perfusion becomes directly proportional to systemic blood pressure. Thus, maintaining systemic blood pressure becomes of paramount importance. Dopamine is most commonly used as a continuous intravenous infusion. The dose should be titrated to achieve the desired hemodynamic and renal response (usually starting at 2-5 mg/kg/min, the dose may be increased to more than 20 mg/kg/min in severe hypotension). Dopamine should be injected into a large vein because extravasation causes tissue necrosis. ECG monitoring is required due to the risk of conduction disturbances. Particular attention should be paid to patients with heart failure.
Cardiac arrhythmias represent a significant risk in severe cases caused by autonomic hyperactivity, metabolic disorders, or the use of high doses of AEDs and/or anesthetics. Continuous ECG monitoring is recommended, and detected arrhythmias are treated in the usual way.
Acute renal or liver failure. Myoglobinuria, disseminated intravascular coagulation, hypotension and hypoxia can lead to renal failure. In the early stages, administration of mannitol and dopamine may be helpful. Acute liver failure can also have various causes, including a hypersensitivity reaction to injected drugs. Drugs to which the patient has a history of hypersensitivity should be avoided.
Other physiological changes. Active treatment is most often required for hypoxia, pulmonary edema and arterial hypertension, cardiac arrhythmias, heart failure, hyperthermia, rhabdomyolysis and disseminated intravascular coagulation. Rhabdomyolysis can be prevented with mechanical ventilation and the use of muscle relaxants.
Identifying the cause of the status. The outcome of the status largely depends on its etiology, and prompt treatment of the cause of ES is vital. CT, MRI and CSF analysis are commonly used, but the choice of tests depends on the clinical situation. If SE was provoked by AED withdrawal, re-prescribing the drug even at a low dose, as a rule, allows you to quickly stop the status. Intravenous pyridoxine is indicated for children under 3 years of age with a current history of epilepsy and for all newborns.
Resuscitation aid and EEG monitoring. If seizures continue despite proper initial treatment, the patient should be transferred to the intensive care unit, where continuous advanced monitoring of vital functions, including intra-arterial and central venous pressure, as well as capnography and oximetry, is indicated. As noted above, electrical seizure activity in the brain can continue even after muscle cramps have subsided and is potentially destructive to cortical neurons. To suppress it, systemic anesthetics are used, reducing the electrical activity of the cortex to the “burst-suppression” phenomenon with a target interval between “flares” from 8 to 20 s. EEG monitoring should begin no later than 1 hour after the onset of the status and continue for at least 24 hours after the cessation of EEG seizure activity.
Increased ICP and its monitoring. If there are signs of persistent, sharply elevated or progressively increasing ICP, then monitoring is necessary. The need for it, as a rule, is determined by the cause of the status, and not by the status itself; ICP monitoring is more common in pediatric practice. If ICP is critically elevated, intermittent positive pressure ventilation, high-dose corticosteroids (4 mg dexamethasone every 6 hours), or mannitol infusions (the latter usually used only in extreme cases as a temporary measure) are used. Neurosurgical decompression is used only in rare situations.
Hyperthermia is the most important cause of poor prognosis of all the systemic disorders that occur in the early stage of PSES. Temperatures above 41 °C lead to damage to hippocampal neurons; in addition, hyperthermia lowers the seizure threshold. All patients with PSES are advised to monitor body temperature and provide passive cooling if it increases.
Bladder catheterization is indicated within the first hour after the onset of the status to assess the volume of fluid excreted. To maintain water-electrolyte balance, intravenous administration of solutions through a venous catheter is indicated.
Specific therapy for GSES
Introduction. The goal of treatment for ES is the fastest possible cessation of its clinical and EEP manifestations and the prevention of its relapse. Treatment for PSES can be divided into several steps depending on the duration of SE. For each of these steps, only drugs available in Russia will be considered.
Initial therapy for GSES can be carried out by the patient himself, his relatives or others, as well as emergency physicians. It should start as early as possible. Benzodiazepines are the drugs of choice. In Russia, diazepam is traditionally used, which is administered intramuscularly or intravenously. The disadvantage of the drug is its short duration of action (no more than 15–30 minutes), as well as its large volume of distribution and tendency to cumulate. Within 2 hours after diazepam administration, ES recurs in patients in more than 50% of cases. Due to its short period of action, diazepam is contraindicated in patients with discrete, recurrent attacks. A more effective alternative to diazepam is midazolam 15 mg IM.
Initial maintenance therapy begins immediately after the administration of benzodiazepines until the results of this administration are obtained (in the event that the SE has resolved after the administration of benzodiazepines, this therapy insures against its recurrence; if the status continues, it serves as a means of stopping it).
Valproate is administered intravenously at a rate of 36 mg/kg/min to achieve a target dose of 25 mg/kg
Alternative: Levetiracetam 1000–3000 mg/day IV at a rate of 2–5 mg/kg/min.
If there is no effect from initial and/or initial maintenance therapy, the patient is diagnosed with refractory ES.
Treatment of refractory SE is usually carried out in the intensive care unit. The patient needs constant monitoring of the EEP, since the dose and rate of drug administration depend not on the clinical manifestations, but on the picture of the EEP.
Sodium thiopental is administered intravenously as a bolus of 100–250 mg over 20 s, then 50 mg every 2–3 min until the seizures stop, then continuously infused at a rate of 3–5 mg/kg/h until the “burst-suppression” phenomenon is achieved. EEG.
Alternative: propofol is started with an IV bolus of 2 mg/kg, repeated if necessary, then infused at 5–10 mg/kg/h until a burst-suppression phenomenon is achieved on the EEG, after which the dose is reduced to a maintenance dose (usually 1– 3 mg/kg/h).
Alternative: intravenous midazolam bolus at a dose of 0.10.3 mg/kg at a rate not exceeding 4 mg/min, followed by continuous infusion to achieve the “burst-suppression” phenomenon on the EEG (usually 0.05–0.4 mg /kg/h).
All drugs for the treatment of refractory epilepsy can depress respiration and heart rate and lead to severe hypotension. Continuous cardiac monitoring, connection to mechanical ventilation, and intravenous administration of dopamine are indicated in patients with hypotension.
Controlled anesthesia with the drugs described above continues for 24–48 hours (in parallel, intravenous administration of AEDs in an average therapeutic dose is carried out), after which the dose of the systemic anesthetic is gradually reduced under the control of continuous EEG monitoring. In case of recurrence of clinical or EEG manifestations of ES, the dose of anesthetic is again increased until the “burst-suppression” phenomenon is achieved on the EEG
The duration of controlled anesthesia in patients with ES can reach several weeks.
Treatment of super-refractory ES is required if GSES is continued within 24 hours after the start of systemic anesthetic administration. If one anesthetic is ineffective, it can be replaced with another. In parallel, AEDs should be administered intravenously or through a nasogastric tube.
Surgical treatment is used for structural lesions that are the cause of HSES. Resection methods or subpial incisions are used. Similar treatment methods are described mainly in pediatric practice.
Immunomodulatory therapy is used for the appropriate cause of the status (anti-NMDA encephalitis, Rasmussen's encephalitis, epilepsia partialis continua). Intravenous glucocorticoids and/or intravenous human immunoglobulin are used.
Hypothermia is a promising way to treat SE and prevent neuronal death. The effectiveness of hypothermia in anoxic status that occurs after cardiac arrest has been described.
Alternative medications, according to isolated observations, can be effective in HSES. These include lidocaine, lacosamide, iso-flurane, etomidate, magnesium sulfate, pyridoxine and a number of others.
Non-drug methods of helping with epilepsy include stimulation of the vagus nerve (single descriptions in children with catastrophic epilepsy), deep brain stimulation, transcranial magnetic stimulation (single descriptions, mainly for epilepsia partialis continua), electroconvulsive therapy, ketogenic diet (for Landau syndrome). Kleffner and some other pediatric syndromes), as well as drainage of cerebrospinal fluid.
Causes of development of convulsive syndrome in children
Usually, the development of seizures is caused by the presence of various diseases or brain injuries, but in some cases, children experience febrile seizures, for which emergency assistance must be called immediately. A similar process is caused by an increase in body temperature. Since brain cells are not properly developed, they perceive overheating as a pathological danger and provoke muscle tone.
Diseases that cause seizures include:
- epilepsy;
- development of inflammatory processes at the brain level;
- post-traumatic state;
- complicated genetic background;
- disturbances in the functionality of the thyroid gland;
- receiving a birth injury to the brain or hypoxia in the case of infants;
- perinatal encephalopathy, which is diagnosed in children under one year of age;
- the presence of an infectious disease in the brain.
When a convulsive syndrome appears in children, it is important to find out the cause of the problem first, since the treatment method depends on it. For this purpose, among other things, bioresonance diagnostics is used, which makes it possible to comprehensively assess all existing disorders and predict possible ones.
Mechanism of seizure occurrence
Convulsive syndrome occurs against the background of unfavorable factors of the internal and external environment, and is especially common in idiopathic epilepsy (epileptic seizure). The development of convulsive syndrome can also be provoked by:
- traumatic brain injuries;
- congenital and acquired diseases of the central nervous system;
- alcohol addiction;
- benign and malignant tumors of the central nervous system;
- high fever and intoxication.
Disturbances in the functioning of the brain are manifested by paroxysmal activity of neurons, due to which the patient experiences repeated attacks of clonic, tonic or clonic-tonic seizures. Partial seizures occur when neurons in one area are affected (they can be localized using electroencephalography). Such violations can occur for any of the above reasons. However, in some cases, when making a diagnosis, it is not possible to accurately identify the cause of this severe pathological condition.
What should parents do if their child has seizures?
If the baby experiences involuntary muscle contractions, then first of all you need to call an ambulance. It is strictly forbidden to carry a child on your own, as this can worsen the baby’s condition. You can’t shake him: you can’t bring him back to his senses this way, but you can easily harm him. While waiting for specialists, the child must be undressed.
If the seizures are not provoked by epilepsy, then you should not unclench the child’s teeth. It is necessary to remove objects that are dangerous to children's lives and open the window for fresh air.
It is strictly forbidden to leave children unattended in this condition.
Relatives should stay nearby until his condition improves. Consultation with a doctor: from 1500 to 4000 rubles Consultation with a chief physician: 4000 rubles
Treatment of seizures in children at the Doctor Choi Clinic
After a baby has been diagnosed with seizures, he is usually registered with a neurologist. The specialist takes the necessary measures to diagnose seizures in a child to identify the factors that provoked their occurrence.
If convulsions no longer recur, then there is no point in treatment; high-quality prevention and general strengthening of the little patient’s body are sufficient. However, if a relapse is diagnosed, drug treatment will most likely be prescribed.
Anticonvulsant therapy is usually used to get rid of a dangerous symptom. It is forbidden to self-treat seizures in children, since uncontrolled use of drugs can lead to a decrease in brain function.
You can avoid taking medications and at the same time prevent the development of the problem by contacting the Doctor Choi clinic. Our specialists have extensive experience in diagnosing and treating children of various ages and have modern equipment and tools. A unique set of strengthening practices will allow the child to quickly feel completely healthy, and most importantly, will protect him from complications associated with drug treatment for seizures in children.
You can obtain more detailed information by making an initial appointment at our clinic.