Group of neurodegenerative diseases
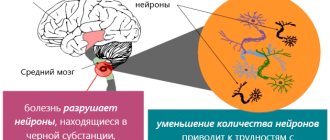
This group of diseases is characterized by damage to the central nervous system. A certain number of neurons die, and this leads to neurological pathologies. Neurodegenerative diseases can have different causes and different clinical manifestations. It is characteristic that all diseases in this category are incurable.
All therapy used is symptomatic. That is, it alleviates the manifestation of the disease, but does not in any way contribute to the regeneration of damaged areas. The most effective would be a transplantation of nerve tissue, but the method has both technical and moral obstacles. Similar diseases include Alzheimer's, Parkinson's, and Farah's diseases.
https://www.youtube.com/watch?v=https:accounts.google.comServiceLogin
A group of similar diseases is characterized by manifestations of central nervous system lesions. A certain number of neurons die, which leads to the development of neurological pathologies. Neurodegenerative diseases can occur for a variety of reasons and also have different clinical manifestations. A characteristic feature of all diseases from this group is their incurability. All therapeutic actions performed are symptomatic.
That is, therapy is aimed at alleviating the symptoms of the disease, but in no way can affect the regeneration of areas that are damaged. The most effective treatment would be tissue transplantation, but this method has several limitations, primarily technical and moral.
The most famous diseases of this group are Alzheimer's disease, Parkinson's disease, and Fahr's syndrome. Early diagnosis of these diseases is often difficult. In the diagnostic process, doctors mainly take into account patients' complaints and observe their behavior. The most modern and effective method for diagnosing diseases of this group is tomography, both computed tomography and magnetic resonance imaging.
Symptoms inherent in the disease
The very first signs of the disease are:
chronic fatigue, fatigue even with reduced physical activity;- lack of coordination during movements, changes in gait (shuffling movements of the legs), clumsiness;
- speech dysfunction;
- the appearance of uncontrolled spastic movements, night muscle activity;
- in childhood, spasms of the fingers and stiffening of the body muscles are observed, giving unnatural postures;
- in adulthood, numbness of the facial areas often occurs, sensory perception and response to external stimuli are slowed down, and movements of all parts of the body are constrained;
- there is persistent tremors in the limbs.
Symptoms of parkinsonism often appear:
- wide, sweeping movements incommensurate with the intended actions;
- weakness in the arms and legs, uncontrolled reflexes in the tendon apparatus;
- loss of control over excretory functions, urinary incontinence;
- in childhood, the manifestation of epileptic syndrome is possible.
Cognitive impairment:
- decline in memory ability;
- slowness of the thought process;
- lack of ability to maintain attention on one process;
- inability to analyze what is happening, decline in intelligence.
The progressive development of the described symptoms leads to the development of oligophrenia in childhood and to dementia in adulthood, however, there are cases when the disease does not lead to a decrease in intelligence in children and adolescents.
Inheritance of Fahr syndrome
Since this disease is a rather rare phenomenon, it is difficult for specialists to provide the correct clinical picture of the syndrome. Symptoms may appear if the patient is over 40 years of age. In younger people, Farah's disease symptoms may be vague and vague. Patients have impaired coordination of movement and parkinsonism.
Also, the development of the disease may be indicated by such signs as tremor, dystonia, rapid and erratic movements of the limbs (chorea), involuntary contractions of the hands and feet. Since the disease affects areas of the brain, performance and mental capabilities decrease. A person’s memory also suffers.
Speech is often impaired. Other neurological signs include pain and mental disorders. There is a juvenile form of the disease, which manifests itself in children and adolescents. The main symptoms are: dystonia, chorea, epileptic seizures. The senile form is typical for middle-aged and older people. In this case, parkinsonism, speech disorders, and other problems in the functioning of the nervous system are observed. Urinary incontinence may also occur.
The reasons why the disease may develop have not been precisely established. However, it is known that disorders of the thyroid and parathyroid glands have a huge impact on its occurrence. In this case, disruptions occur in the metabolic processes of calcium and phosphorus. Similar neurodegenerative diseases can also occur when the acid-base balance in the body is disturbed.
Description and causes of the disease
The pathology is characterized by symmetrical deposition of calcium elements in the structures of the brain - in the subcortical region, meninges and cerebellar zone. The disease includes only cases of primary calcification; mineral accumulations against the background of other pathologies (secondary cases) are not included in the clinical picture of the disease. For the first time, the most complete description of the anomalous process was created in the 30s of the last century by the German doctor K.T. Farom.
The disease is classified as rare, as only one case is recorded per million people on the planet. The male half of humanity is susceptible to these disorders twice as often as the female half. The phenomenon can appear at any age, but the most common age category is the period from 30 to 60 years. Since the disease is practically asymptomatic, and obvious signs are very similar to other neurological diseases, only about 2% of pathological cases are detected during the patient’s lifetime.
External and internal factors in the development of the disease have not yet been established. Most medical scientists tend to believe that there is a congenital, genetic etiology, since a hereditary factor can often be traced in the same family. There are studies that prove the theory of mutations in the genetic code affecting chromosomes 14, 2 and 8; this issue is still being studied. This theory is counterbalanced by cases where the disease manifested itself in people who did not have relatives in the older generation with this phenomenon. The first manifestation of symptoms occurs already at the stage of critical filling of the brain fibers with calcium inclusions, which does not allow studying the course of the disease during a person’s lifetime.
Pathological studies reveal the symmetrical presence of calcifications in the tissues of the frontal lobes and the cerebellar zone. Most of the accumulations are present in the subcortical region, less often deposits are seen on the vascular canals (mostly on the arterial walls). In appearance, the deposits resemble microscopic thread-like channels interspersed with metal elements of various types.
In the medical literature, there are two forms of pathology, divided by age and inherent symptoms. The first form is represented by the so-called juvenile form, since primary manifestations occur in childhood or early adolescence. There is a slight decline in intellectual and cognitive abilities, and a slowdown in the functionality of the muscular system. A frequent “accompanying” of this form of anomaly is parallel mental retardation. Over time, symptoms develop into signs of Parkinson's disease.
The second form is characterized as cyanide and manifests itself in middle or old age. The main symptoms are signs of parkinsonism with obvious impairments in the decline of intellectual abilities. Over time, profound dementia progresses.
Diagnostic methods
Before the advent of computed tomography, patients underwent X-ray examinations of the brain. Thanks to the advent of modern research methods, specialists received more informative pictures from the affected area. However, it is worth noting that computed tomography rather than magnetic resonance imaging has greater sensitivity for this clinical picture.
When analyzing most images, the affected areas are limited to the globus pallidus. They are small in size. Most often, the basal ganglia, thalamus, and cerebellum undergo changes. The level of calcification is approximately the same in young and elderly people. Also, no differences were found between groups in which Farah's disease is asymptomatic and those in which the syndrome is accompanied by pronounced symptoms.
When conducting a postmortem examination, the following picture is observed in the brain: the vessels have a whitish appearance (some branched areas). When touched by a knife, they make a sound similar to a crunch. For histological analysis, material is collected: sections of the cerebral cortex, basal ganglia, and cerebellum.
It is in the area of the latter that calcification occurs most often. The samples contain calcium salts. They are also most often detected on arteries (small, medium-sized) and capillaries. Less commonly (in isolated cases), Farah's disease affects the veins. Small calcium conglomerates are found along the entire length of the vessels, as well as in adjacent tissues. The presence of traces of arsenic, aluminum, cobalt, and mucopolysaccharides is also detected in the tissues.
Depending on whether Farah disease has symptoms, making a diagnosis can be somewhat more difficult. It is often diagnosed accidentally, when a tomography is performed to confirm a completely different disease. First of all, the specialist excludes calcium metabolism disorders and other developmental defects. Then a computed tomography scan (or x-ray examination) is prescribed.
Doctors note that one of the problems in making a correct diagnosis is hypoparathyroidism. This is a disease that occurs due to parathyroid hormone deficiency. As a result, the level of calcium in the blood decreases, and the level of phosphorus increases. If calcification of the striopallidodentate structures is observed on a CT scan, then additional tests are necessary to distinguish hypoparathyroidism from a condition such as Fahr's disease.
Prevention of hypoparathyroidism
To prevent the occurrence of hypoparathyroidism during surgical operations on the thyroid gland, gentle surgical techniques should be used in relation to the parathyroid glands. In patients with recurrent toxic goiter, it is recommended to undergo radiation therapy with radioactive iodine instead of surgery to avoid the development of hypoparathyroidism. It is important to prevent postoperative complications (adhesions, infiltrates), which can disrupt the blood supply to the parathyroid glands, as well as early detection of symptoms of increased neuromuscular excitability in postoperative patients and timely adoption of measures.
To prevent convulsive syndrome (tetany) and the development of acute hypocalcemic crisis in hypoparathyroidism, it is necessary to exclude provoking factors and prevent intoxication and infections.
Patients with hypoparathyroidism are required to follow a dairy-vegetable diet, enriched in calcium and depleted in phosphorus, without meat products that cause the development of tetany, and also be registered with a dispensary to monitor the content of calcium and phosphorus in the bones and blood.
Consequences
What are the consequences of Farah disease?
All of the listed characteristics are realized only if there is complete dominance, and for the appearance of the trait, only one gene of a dominant nature will be sufficient.
https://www.youtube.com/watch?v=ytaboutru
Based on the listed features of the type of inheritance through which Farah syndrome is transmitted, we can say that the presence of a diseased gene in the future father can cause the disease to manifest itself in the child. In order to determine the likelihood with which your child may inherit Farah disease, you should contact a genetics specialist who will conduct all the necessary studies and calculations and give a fairly accurate result.
Clinic and symptoms
Determining the presence of Farah's disease is complicated by an unclear clinical picture. The disease is often asymptomatic. Even if a person has been diagnosed with the development of the syndrome, he often does not feel it. Pathological accumulation of calcium in the brain area is manifested by the following symptoms:
- motor dysfunction;
- deterioration of mental activity, up to dementia;
- spastic paralysis;
- headache;
- disturbances in the movement of the eyeball;
- dysarthria;
- convulsive syndrome.
When Fahr syndrome manifests itself with signs of parkinsonism, its symptoms may be:
- tremor;
- constant presence of muscles in a tense state;
- shuffling gait;
- a motionless face resembling a mask;
- involuntary clenching of fingers, simulating rolling of pills.
Such symptoms are characteristic of the late stage of the disease. In addition to the neurological and mental symptoms of the disease, signs of skull abnormalities, glaucoma, retinitis pigmentosa, and pathology of the endocrine system (hypoparathyroidism) are possible, although much less common.
Forms of the disorder - their symptoms
Common to any neurodegenerative disease is the slowly progressive death of nerve cells, but it can manifest itself in different forms and varieties.
The most common manifestation is parakinsonism, increased muscle rigidity. Dysfunction of the parathyroid gland, either primary or after surgery, alters the production of parathyroid hormone, which in turn increases the level of phosphorus in the blood and decreases the level of calcium.
Such disorders can manifest themselves in the form of tremor, dystonia, dyskinesia, involuntary spasms of the face, limbs or torso.
The next most common are disorders of the basic functions of the brain, which are manifested by weakening of memory, an excessively strong reaction to various events in a person’s life.
The connection of the disease with a disruption of normal calcium metabolism leads to neurological manifestations, accompanied by muscle spasms. The disease also manifests itself in psychiatric disorders, changes in approach, and severe pain.
Fahr syndrome is often diagnosed against the background of diseases such as toxoplasmosis, infection by the pork tapeworm parasite, and echinococcosis. Less commonly diagnosed is the disease expressed as tuberous sclerosis of Bourneville.
Privileged body
The brain is an organ that is remarkable in many ways [1]. The infinite complexity of the device, its functionality and the connection between our lives and its state attract the attention of researchers to the brain. The relationship between the brain and the immune system of our body is also special: the brain is an immune-privileged organ. Immune reactions that easily develop in other tissues (liver cells, muscles, fatty tissue) rarely occur in the brain. Along with the brain, the thyroid gland, testicles and some eye tissues, in particular the cornea, found themselves in such a special relationship with the immune system.
In the minds of doctors in the second half of the twentieth century, the brain was a structure completely isolated from the immune processes of the rest of the body. At the beginning of the last century, Japanese scientists conducted experiments on implanting sarcoma cells (a malignant tumor of muscle tissue) into various organs of mice [2]. It turned out that when sarcoma cells are placed in brain tissue, the tumor actively develops, but when they are implanted under the skin of rodents or into muscle tissue, the tumor does not develop. The researchers suggested that the “center” (the brain) is isolated from the immune processes that successfully fought off the sarcoma “in the periphery” (other tissues). Further research only confirmed this conclusion. The brain, in the minds of scientists, has become a “clean zone” in which an immune response does not develop. In the 1950s, the term “immunological privilege” arose, coined by British scientists named Billingham and Boswell [3].
Overcoming barriers
It has long been believed that the basis of the immune privilege of the brain is the presence of the blood-brain barrier (BBB). The BBB is a complex of cellular and extracellular structures that separate the blood flowing in the capillaries from the neurons of the brain parenchyma. The cells of the vascular walls, the basement membrane on which they lie, and astrocytes participate in the formation of the BBB. The immune privilege of parts of the central nervous system was in good agreement with the extent of the BBB within it. The brain parenchyma is reliably protected by the BBB, and inflammatory processes (encephalitis) rarely occur in it. The choroid plexus, which produces cerebrospinal fluid, and the membranes of the brain do not have such cover, and their inflammation (choroiditis, meningitis) is much more common (Fig. 1).
Figure 1. Structure of the central nervous system. The parts that are well protected by the BBB include the gray and white matter. Other components do not have this protection.
[2]
There are cases in which even the BBB does not save the nervous system, and inflammation processes occur in the brain. However, it is important to answer the question for yourself: do they proceed in the same way as in the rest of the body? Maybe the brain has a special way of immune response, and immune privilege has additional explanations? In vitro, an immune response in tissues can be induced by injecting bacterial lipopolysaccharide (LPS). It is a structural component of the cell wall of gram-negative bacteria that triggers a cascade of immune reactions in our body. When studying the reaction of different body tissues to the introduction of LPS, it turned out that an immune response in the skin and choroid plexuses of the central nervous system occurs to the same dose of LPS [4]. This is logical, because in neither case is there special protection in the form of the BBB. At the same time, to cause an immune reaction in the brain parenchyma similar to that occurring in the skin, a dose 100 times greater than the “skin” dose is required. In this case, compartmentalization of the immune response occurs - predominantly the white matter (the processes of neurons) is affected, and not the gray matter (their bodies). This experience points to another explanation for immune privilege: the weakness of our brain's own, “intrinsic” immune response. The brain parenchyma turns out to be extremely passive in relation to bacterial invasion: the brain does not seem to want to notice foreign components inside itself. Next we will try to figure out what is the reason for this immune “inattention”.
Weak link
If we are talking about the weakness of the local immune response, then we need to figure out what is “broken” in it. This may be impaired recognition with the normal ability of immune cells to attack antigens (weakness of the afferent link). Another option: normal antigen recognition with impaired ability to attack it (weakness of the efferent link). Studies have shown that the efferent part of the brain is preserved and functions normally [2]. Then efforts were focused on searching for disorders in the afferent link. Over time, it became clear that the problem was in recognizing the antigen and further working with it (Fig. 2).
Figure 2. The immune response occurs in several stages. The formation of a reaction to an antigen occurs in two ways: information about the antigen is delivered to local lymph nodes in “liquid” (the antigens themselves) and “solid” form (previously activated cellular elements of the immune system). In an immune reaction outside the brain, both pathways operate. When the brain's immune system reacts to an antigen, cellular elements do not reach the local (cervical) lymph nodes. It is one of the main components of a weak immune response in the brain.
[2]
You can read more about these processes in the article “Immunity: the fight against strangers and... our own” [5].
The brain has difficulty delivering activated immune cells to the cervical lymph nodes. Dendritic cells are responsible for working with antigens in the brain. In other organs, they recognize the antigen and then provide information about it to T and B lymphocytes in the lymph nodes. During inflammation in the brain, this process does not occur: dendritic cells do not migrate to the lymph nodes and do not present antigen. The immune response becomes localized and is regulated by dendritic cells in the brain parenchyma. If in other organs the cellular elements of immunity, after the presentation of the antigen, rush to the site of bacterial penetration [6], then with the development of inflammation in the brain parenchyma this does not happen. The brain has to rely on itself. A similar locality of the immune response is observed in the parenchyma, but not in the choroid plexuses and meninges.
Immigrants in the brain
If we continue to describe the special immune status of the brain in our body, then it is necessary to say a few words about microglia. Microglia are the main cell type responsible for the innate immune response in the brain (Fig. 3) [7]. Microglia have phagocytic activity, are able to recognize microorganisms and contribute to the development of inflammatory reactions in the brain parenchyma, that is, they behave not like a neuron, but a real immune cell [8]. This is explained by the fact that microglia in our brain are of hematopoietic origin [9]. The precursors of microglia in the prenatal period are the “brothers” of all other immune cells that find themselves in the central nervous system. They did not lose their immune functions and began to carry them out locally. It turns out that microglial cells can be called emigrants of the immune system.
Treatment
In most cases, treatment for the disease is aimed at stopping the progression of vision loss. In a small number of cases (with secondary damage, for example, multiple sclerosis, brain tumors, etc.), lost vision can be partially or completely restored. That is why the greatest importance in the secondary mechanism of development is given to the treatment of the underlying disease.
For treatment, medications, physiotherapy, and therapeutic exercises for the eyes are used. Among the medications used for treatment, angioprotectors, repair stimulants, metabolic and neuroprotective agents are used, and local agents are also used (for example, emoxypine drops). Therapeutic exercises are an auxiliary method, but in some cases they can lead to good results. In some cases, various laser surgery techniques are indicated.
Calcinosis during pregnancy
Calcium deposition during pregnancy is most often diagnosed at the end of the third trimester of the gestational period. From a medical point of view, such a process is permissible and is associated with a modification of the placenta.
If calcification is diagnosed earlier, it can lead to premature maturation of the placenta. As a rule, calcinosis in pregnant women is associated with the consumption of large amounts of foods rich in calcium, infectious processes and metabolic disorders.
An excess of a macronutrient in a pregnant woman’s body is just as dangerous as its deficiency. May cause injury to the baby and mother during delivery.